��Ŀ����
��13�֣�����W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ �أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ����ש��ɫ����
�أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ����ש��ɫ����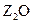 �ͺ�ɫ��ZO���������
�ͺ�ɫ��ZO���������
��1��Wλ��Ԫ�����ڱ���_________���ڵ�_________�塣
W����̬�⻯���ȶ��Ա�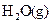 __________���ǿ������������
__________���ǿ������������
��2��Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��
_________________________________________________________________________��
��3��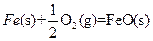
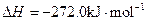

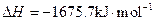
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
������֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ____________ __
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ____________ _
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ_____ ___
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________��
 �أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ����ש��ɫ����
�أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ����ש��ɫ����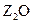 �ͺ�ɫ��ZO���������
�ͺ�ɫ��ZO�����������1��Wλ��Ԫ�����ڱ���_________���ڵ�_________�塣
W����̬�⻯���ȶ��Ա�
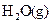 __________���ǿ������������
__________���ǿ��������������2��Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��
_________________________________________________________________________��
��3��
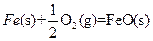
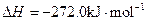

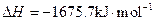
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
������֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ____________ __
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ____________ _
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ_____ ___
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________��
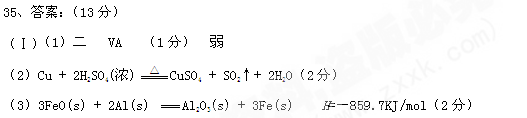
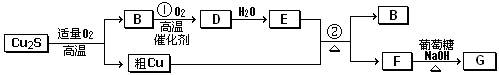
����(1)1s22s22p63s23p63d104s24p3����2�֣�
(2)
 ��2�֣�
��2�֣�(3)�����Σ�1�֣���
(4)As2O3��6Zn��6H2SO4===2AsH3����6ZnSO4��3H2O��2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
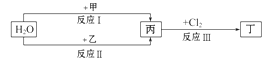
 __________________________________����֤��������ʯ�д���+2����Ԫ�ء�
__________________________________����֤��������ʯ�д���+2����Ԫ�ء� ��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4��
��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4�� ��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____�� ____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
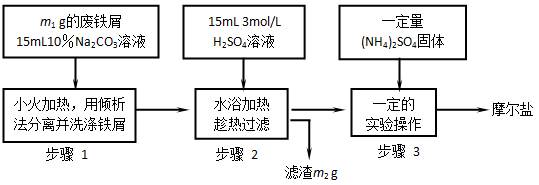
____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��  4��(NH4)2SO4��ҪС����ˮ�⣻���л�ԭ�ԣ��������������ȶ��������ǽ��̷�(FeSO4?7H2O)���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��������ͼ��
4��(NH4)2SO4��ҪС����ˮ�⣻���л�ԭ�ԣ��������������ȶ��������ǽ��̷�(FeSO4?7H2O)���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��������ͼ�� (NH4)2SO4?FeSO4?6H2O����������ͼ�ش�
(NH4)2SO4?FeSO4?6H2O����������ͼ�ش�