��Ŀ����
��15�֣��±���Ԫ�����ڱ���һ���֡�
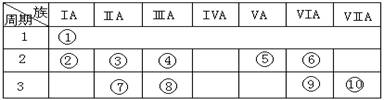
������˵����ȷ����˵��Ԫ�آ�ķǽ����Ա�Ԫ�آ�ǿ��������������������
A��ͬ��ͬѹ�£�Ԫ�آ����̬�⻯���ܽ�ȴ���Ԫ�آ����̬�⻯��
B��Ԫ�ص�����������Ӧ��ˮ��������Ԣ�ǿ�ڢ�
C��Ԫ�آ�͢�ĵ���������Ӧ�ֱ�õ��ͼۡ������Ļ�����
D��Ԫ�آ�ĵ縺�Դ���Ԫ�آ�
��ijԪ��ԭ�ӵĺ���p��������s��������1�����Ԫ�ص�Ԫ�ط����� �����Ԫ�ص��ʷ��ӻ�Ϊ�ȵ�����Ķ����������� ��
����֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آ���������������Ƶ����ʡ�д��Ԫ�آ۵��������������ᷴӦ�����ӷ���ʽ��
���� ��
������Ԫ�آ��γɵľ����Ӧ�ľ���Ϊ��ͼ�е�___________________(��д���)��
�� �� �� ��
��Ԫ�آܵĺ�����Ľṹʽ�ɱ�ʾΪ��������жϸ���Ϊ�����ᣨ��ǿ������
һ���Ӹ�����ˮ���ã�ֻ�ܲ���1��H������д����������ˮ����Һ�����Ե����ӷ���ʽ������������������������������������������������ ��
����֪Ԫ�آ���̬�Ȼ���Ļ�ѧʽΪR2Cl6���ṹʽ���£��Բ�������е���λ��
![]() Cl Cl Cl
Cl Cl Cl
Al Al
Cl Cl Cl
��15�֣�
��B C D (2����ѡ��1��) ��N (2��)C22��(2��) ��Be(OH)2 + 2H+= Be2++ 2H2O(2��)
�� �� (2��)����(1��) H3BO3+H2O H4BO4-+H+ (2��)
![]() �� Cl Cl Cl
�� Cl Cl Cl
Al Al
Cl Cl Cl (2��)
����:




 NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ��
NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ�� ��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����
��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����