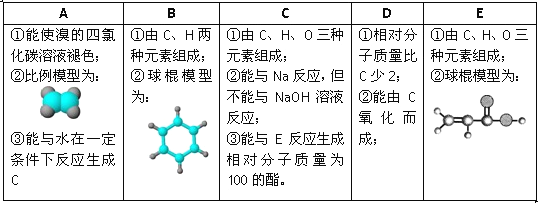
��Ŀ����
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ���� (�� ��)
A. ��״���£�16g�ļ״�����������������10NA
B. ��״���£�22.4 L�����й��ۼ���ĿΪ19NA
C. 1 mol���ͱ�����Ļ������ȫȼ��ʱ����O2�ķ�����Ϊ7.5NA
D. 0.3 mol AgNO3������ȫ�ֽ�(2AgNO3===2Ag��2NO2����O2��)������ˮ���ռ�������ķ�����Ϊ0.25NA
���𰸡�C
��������A. 16g�ļ״������ʵ���Ϊ![]() =0.5mol������9mol���ӣ���A����B. ��״���£����鲻�����壬������22.4 L�����й��ۼ�����Ŀ����B����C. 1 mol���ĺ�������1mol������ĺ�������ȣ�1 mol���ͱ�����Ļ������ȫȼ������O2�����ʵ���Ϊ6+
=0.5mol������9mol���ӣ���A����B. ��״���£����鲻�����壬������22.4 L�����й��ۼ�����Ŀ����B����C. 1 mol���ĺ�������1mol������ĺ�������ȣ�1 mol���ͱ�����Ļ������ȫȼ������O2�����ʵ���Ϊ6+![]() =7.5mol����C��ȷ��D. �����ɵ�����NO2��O2ͨ��ˮ�к�����Ӧ��4NO2+O2+2H2O=4HNO3������0.3molAgNO3������ȫ�ֽ����ɵ�������NO2��O2�����ʵ����ֱ�Ϊ0.3mol��0.15mol������ˮ���ռ�����������0.075mol����ʣ��0.15mol-0.075mol=0.075mol�����ռ����ķ�����Ϊ0.075NA������D����ѡC��
=7.5mol����C��ȷ��D. �����ɵ�����NO2��O2ͨ��ˮ�к�����Ӧ��4NO2+O2+2H2O=4HNO3������0.3molAgNO3������ȫ�ֽ����ɵ�������NO2��O2�����ʵ����ֱ�Ϊ0.3mol��0.15mol������ˮ���ռ�����������0.075mol����ʣ��0.15mol-0.075mol=0.075mol�����ռ����ķ�����Ϊ0.075NA������D����ѡC��

����Ŀ����������Ϊ����ʹ�õ�ʳ�����ϣ��ֳ������������������Ĵ̼�ζ�Ͳ������Ĺ�������������ǿ����ķ�������������Դ���ζ��
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH
���й��л���ķе�����
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�(��) | 34.7 | 78.5 | 100.5 | 54.4 |
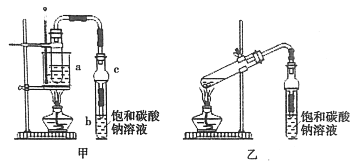
��1���Ʊ���Ʒ
��4mL�Ҵ���3mL�����2mLŨ��������Թ�a�У�ҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�b�ڵõ����������Ĵ�Ʒ��
��ʵ��ʱ����������Ũ���ἴ��������ã���ʵ���������ڴ�����ԭ����__________��Ũ���������ֲ��ܹ��࣬ԭ����_________��
��������C��������__________��
��������װ���Ʊ�������������ȱ����__________��
(2)�Ʊ���Ʒ
�ٷ�Ӧ�������Թ�b�з��������������Ʒ���õ���Ҫ����__________��
�ڴ�b�з�����ļ��������г������������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ���ȥ______��Ȼ����ͨ��_________�����õ�����������
��ijͬѧ��װ�б����������Ƶ��Թ��ռ���������������û���ռ������ԭ����______________���û�ѧ����ʽ���ͣ���