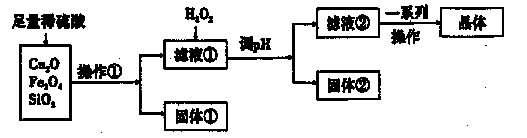
��Ŀ����
�����������壨FeSO4·7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����������ò�Ѫ���Ƿ���ʡ�ʵ�鲽�����£�
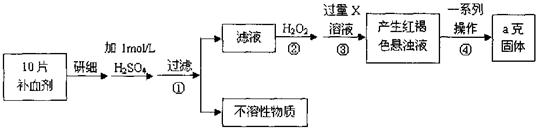
��ش��������⣺
��1���������Һ�еμ�KSCN��Һ����Һ��Ϊ��ɫ�������Һ�к��� (�����ӷ���)��������Һ�л�����Fe2+�ķ���Ϊ
��ע���Լ�������
��2������ڼ������H2O2��Ŀ���� ��
��3��������з�Ӧ�����ӷ���ʽΪ ��
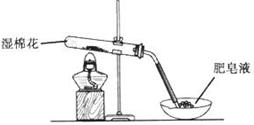
��4���������һϵ�д����IJ���������������ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g��

��ش��������⣺
��1���������Һ�еμ�KSCN��Һ����Һ��Ϊ��ɫ�������Һ�к��� (�����ӷ���)��������Һ�л�����Fe2+�ķ���Ϊ
��ע���Լ�������
��2������ڼ������H2O2��Ŀ���� ��
��3��������з�Ӧ�����ӷ���ʽΪ ��
��4���������һϵ�д����IJ���������������ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g��
��1��Fe3+ ȡһ������Һ���μ�����KMnO4��Һ��KMnO4��Һ��ɫ
��2����Fe2+ȫ������ΪFe3+
��3��Fe3++3OH��= Fe��OH��3������Fe3++3NH3·H2O = Fe��OH��3+3NH4+��
��4��ϴ�� ��ȴ
��5��0.07a (��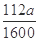 ���仯����ʽ����
���仯����ʽ����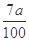 )
)
��2����Fe2+ȫ������ΪFe3+
��3��Fe3++3OH��= Fe��OH��3������Fe3++3NH3·H2O = Fe��OH��3+3NH4+��
��4��ϴ�� ��ȴ
��5��0.07a (��
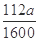 ���仯����ʽ����
���仯����ʽ����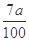 )
)�����������1��KSCN��Һ��Fe3+��Ӧ��Ѫ��ɫ����Fe3+���ڵ�����������Fe2+�Ļ�ԭ�Լ�������ڣ�ȡһ������Һ���μ�����KMnO4��Һ��KMnO4��Һ��ɫ����2��H2O2�ǰ�Fe2+����ΪFe3+��������Ϊ�˽�Fe2+ȫ������ΪFe3+����3������Һ�м���ǿ����Һ��������������������4��Ϊ�˵õ�����������������Ҫϴ�ӣ����գ���ȴ��õ�����5������Ϊ��������10Ƭ��Ѫ���õ��ģ�ÿƬ��Ѫ��������Ԫ�ص�����Ϊa��160��56��2��10="0.07a" g��

��ϰ��ϵ�д�
�����Ŀ

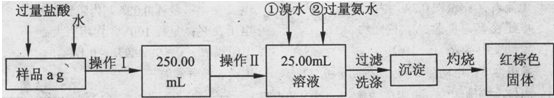
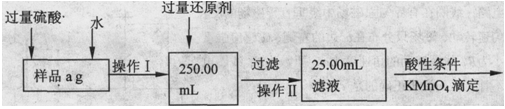
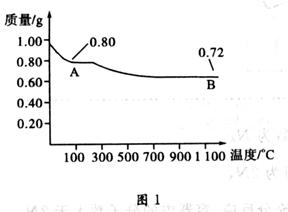

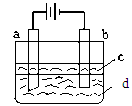
 2FeCl2��CuCl2������FeCl3��Һ�м���a g Cu�ۣ���ȫ�ܽ���ټ���b g Fe�ۣ���ַ�Ӧ������c g������塣��c��a��������˵����ȷ����
2FeCl2��CuCl2������FeCl3��Һ�м���a g Cu�ۣ���ȫ�ܽ���ټ���b g Fe�ۣ���ַ�Ӧ������c g������塣��c��a��������˵����ȷ����