��Ŀ����
3����a gͭþ�����Ͻ��ܽ���һ������Ũ�����У����Ͻ���ȫ�ܽ�ʱ���ռ���NO��NO2��N2O4��N2O������壮�û���������״����V L O2��ֻ��ͨ��ˮ��ǡ����ȫ��ˮ���գ�����Ӧ�����Һ�м�������İ�ˮ���õ������������غ㷨��������������õ����غ��ϵ�����ǣ�������| A�� | �����غ㡢�����غ㡢����غ� | B�� | �����غ㡢�����غ㡢����غ� | ||
| C�� | �����غ㡢����غ㡢�����غ� | D�� | ����غ㡢�����غ㡢�����غ� |
���� �ռ�����NO��NO2��N2O4��N2O����������״����V L O2��ֻ��ͨ��ˮ��ǡ����ȫ��ˮ���գ����ݵ����غ��֪�������õ��ĵ��ӵ����ʵ����������������Ӧ��������ʱ�õ����ӵ����ʵ�����ȣ����յõ�����Ϊ�������������������=��������+��������������������ʧȥ�������ϵ����������ӵ����ʵ�����ȣ����õ���غ�ɼ�������������ӵ����ʵ����������������غ㶨�ɼ�����õ�������������
��� �⣺���ռ�����NO��NO2��N2O4��N2O����������״����V L O2��ֻ��ͨ��ˮ��ǡ����ȫ��ˮ���գ����ݵ����غ��֪�������õ��ĵ��ӵ����ʵ����������������Ӧ��������ʱ�õ����ӵ����ʵ�����ȣ�
�����յõ�����Ϊ��������������������������������=��������+�����������������������������=���������ӵ����ʵ���=����ʧȥ���ӵ����ʵ��������õ���غ�ɼ�������������ӵ����ʵ�����
��������������غ㶨�ɼ�����õ�������������
�����õ����غ��ϵ�ֱ�Ϊ�������غ㡢����غ㡢�����غ㣬
��ѡC��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ����غ㡢�����غ㶨�ɡ������غ���ڻ�ѧ�����е�Ӧ�÷���Ϊ���ؼ���������ؿ���ѧ���ķ�����������������ѧ����������

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�| A�� | ����Һ�����屽�ķ�Ӧ��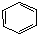 +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$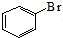 +HBr +HBr | |
| B�� | �������Ҵ���������Ӧ��CH3COOH+CH3CH2${\;}_{\;}^{18}$OH$��_{��}^{ŨH_{2}SO_{4}}$CH3CO${\;}_{\;}^{18}$OCH2CH3+H2O | |
| C�� | ��������NaOHˮ��Һ����� CH3CH2Br+NaOH$��_{��}^{ˮ}$CH2=CH2+H2O+NaBr | |
| D�� | �Ҵ�����ȥ��Ӧ CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O |
| A�� | ��ȥ���������е�������������Ҵ���Ũ���ᣬʹ����ȫ��ת��Ϊ�������� | |
| B�� | ��ȥ���е��������ӣ�����NaOH��Һ�������÷ֲ��ȥˮ�� | |
| C�� | ����������Ӧ���Թܣ�����ϡ����ϴ�� | |
| D�� | ��ȡ�ܽ���ˮ�е������⣺����CCl4�������÷ֲ��ȡ���л����ٷ��� |
| A�� | 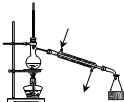 ��������ʯ�� | B�� |  �����ռ�NO | ||
| C�� |  ���ڷ��������������������� | D�� | 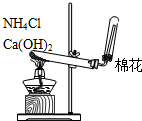 ����ʵ������ȡNH3 |
| A�� | ���ʵ�Ħ����������1mol���ʵ����� | |
| B�� | 1molˮ��������ˮ��Ħ��������ˮ����Է�����������ֵ�϶���18 | |
| C�� | �������ʵ�Ħ��������������ͬ | |
| D�� | �����ʵ����������ʵ��������أ�������Է�������һ���������ʵĻ������� |
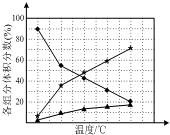 ��1��������N2H4����һ�ָ���ȼ�ϣ���ҵ�Ͽ������õ����������Ʊ�������
��1��������N2H4����һ�ָ���ȼ�ϣ���ҵ�Ͽ������õ����������Ʊ�������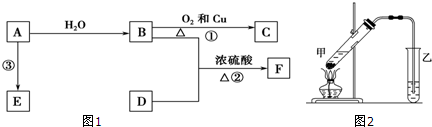
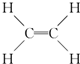 ��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���
��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���