��Ŀ����
����Ŀ����Ԫ�����γ�NH3��NO2��HNO3�ȶ��ֻ����
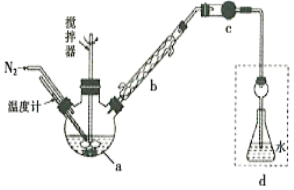
��1��ʵ������ȡ�����Ļ�ѧ����ʽΪ___________________________����ˮ���ն���İ���ʱ���罫����ֱ�Ӳ�����ˮ�У�����������������������ԭ��Ϊ_________________��
��2�������ǹ�ҵ�Ʊ������ԭ�ϣ��ù����е�һ����Ӧ�Ļ�ѧ����ʽΪ__________��
��3�������ΪVmL���Թܳ���NO2����ˮ�У�����ͼ����ַ�Ӧ���Թ��е���Һ���Ϊ__________����ʹ�Թ���NO2��ȫ�����գ������Թ�����Һ�����ʲ���ˮ������ɢ����������еIJ���Ϊ_____________��
���𰸡�2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O������������ˮ��ʹװ���е�ѹǿС������ѹǿ��������4NH3+5O2
CaCl2+2NH3��+2H2O������������ˮ��ʹװ���е�ѹǿС������ѹǿ��������4NH3+5O2![]() 4NO+6H2O2V/3 mL�ٻ���ͨ��V/4 mL����
4NO+6H2O2V/3 mL�ٻ���ͨ��V/4 mL����
��������
��1��ʵ������ȡ�������õ�����ʯ�Һ��Ȼ�炙�ϼ��ȣ���Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O�����ڰ�����������ˮ���罫����ֱ�Ӳ�����ˮ�У�ʹװ���е�ѹǿС������ѹǿ���Ӷ�����������
CaCl2+2NH3��+2H2O�����ڰ�����������ˮ���罫����ֱ�Ӳ�����ˮ�У�ʹװ���е�ѹǿС������ѹǿ���Ӷ�����������
��2�������ǹ�ҵ�Ʊ������ԭ�ϣ��ù����е�һ����Ӧ�ǰ��Ĵ���������Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
��3�������ΪVmL���Թܳ���NO2����ˮ�У�������Ӧ3NO2+H2O��2HNO3+NO����˳�ַ�Ӧ��ʣ��![]() ��NO�����Թ�����Һ�����Ϊ
��NO�����Թ�����Һ�����Ϊ![]() ������4NO+3O2+2H2O��4HNO3��֪��ʹ�Թ���NO2��ȫ�����գ���Ҫ��ͨ�������������Ϊ
������4NO+3O2+2H2O��4HNO3��֪��ʹ�Թ���NO2��ȫ�����գ���Ҫ��ͨ�������������Ϊ![]() ����������еIJ���Ϊ�ٻ���ͨ��
����������еIJ���Ϊ�ٻ���ͨ��![]() ������
������