��Ŀ����
����Ŀ��ijͬѧ����480 mL 0.5 mol/L NaOH��Һ��
�� ��ͬѧ��ʵ������У��õ��IJ��������У���Ͳ������������ͷ�ι�___________��
�� �������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�____(��ѡ����ĸ)֮�䡣
A������� B������� C������� D�������


�� ��ͬѧӦ��ȡNaOH����___g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С_____(��Сд��ĸ)��
������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��____(���д��ĸ)��
������������
a | b | c | d | e | |
�����С/g | 100 | 50 | 20 | 10 | 5 |
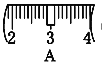

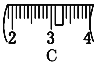
�� ��ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48 mol��L��1��ԭ�������____(�����)��
A������NaOH����ʱ�������ˡ�������� B������ƿ��ԭ����������ˮ
C���ܽ������ձ���Һ��δϴ�� D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶�
���𰸡� �ձ���500mL����ƿ C 10.0 cd C ACD
����������1��ʵ����û��480mL������ƿ������ʱӦ��ѡ��500mL������ƿ�����Ʋ����У����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ��500mL����ƿ���ձ�����2���������������ͼ��ʾ����ͼ��������������ƿ�еμ�����ˮ������Ӧ�ڶ���֮ǰ������ͼ�еĢ����֮�䣬��ѡC����3����ͬѧӦ��ȡNaOH����������0.5L��0.5mol/L��40g/mol��10.0g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ����Ҫ����������30g�������ƶ���3.1g�������Ը��ݱ������ݿ�֪ѡȡ����������Сc��d������ȷ��ʾ����λ�õ�ѡ����C����4����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48 mol��L��1����Ũ��ƫ�͡���A������NaOH����ʱ�������ˡ�������������³������������Ƶ�����ƫС�����Ƶ���Һ���������Ƶ����ʵ���ƫС����ҺŨ��ƫ�ͣ�A��ȷ��B������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�������û��Ӱ�죬���Բ�Ӱ�����ƽ����B����C���ܽ������ձ���Һ��δϴ�ӣ��������Ƶ���Һ�������������Ƶ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�C��ȷ��D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ���������ʱ���������ˮ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ�D��ȷ����ѡACD��

 ��У����ϵ�д�
��У����ϵ�д�