��Ŀ����
����Ŀ�����͵�����ػ������ںܶ��������Ź㷺��Ӧ�á���ش�
I.����������ʮһ�����ij���-2F���ʹ�õ��ƽ���ȼ����N��H����Ԫ����ɣ���ԭ�Ӹ���N:H=1:2����ˮ��Һ�Լ��ԡ�
(1)��������Nԭ�ӵ��ӻ���ʽΪ________������ˮ�ʼ��Ե�ԭ��Ϊ___________(�����ӷ���ʽ��ʾ)��
(2)��Ԫ�صĵ�һ�����ܱ����ڵ���Ԫ�ش���ԭ��Ϊ________________��
II.Ц��(N2O)������������������������ʳ�ᵼ������������ҡ�
(3)Ԥ��N2O�ĽṹʽΪ________________��
(4)�ڶ�����Ԫ����ɵ������У�д����NO2-��Ϊ�ȵ�����ķ���_________��(д�����������ʽ)
III.������Ϊ���ɫ���壬�з½�Ч����Խ��Խ��س�Ϊ�ƽ�Ĵ���Ʒ��
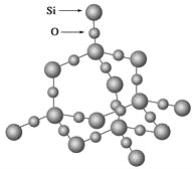
(5)Ti��������Ķѻ�ģ��Ϊ________����λ��Ϊ_______����̬Ti3+��δ�ɶԵ�������______����
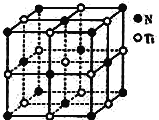
(6)�����Ѿ���ľ�����NaCl��������(��ͼ��ʾ)���õ����ѵ��ܶ�Ϊ��g��cm-3����þ�����N��Ti֮����������Ϊ______nm(NAΪ�����ӵ�������ֵ��ֻ�м���ʽ)���þ������뵪ԭ�Ӿ���������������ԭ��Χ�ɵĿռ伸����Ϊ____________________��
���𰸡�sp3 N2H4��H2ON2H5+��OH- ��ԭ�ӵ�2p���Ϊ2p3������ȶ�״̬�������ٵ�������ӣ��ʵ�ԭ�ӵ�һ�����ܴ�����ԭ�� N=N=O SO2��O3 �������ܶѻ� 12 1 ![]() ��������
��������
��������
(1)�����֪��������N2H4��Nԭ�ӵ��ӻ���ʽΪsp3����ˮ��Һ�Լ���ԭ����NH3���ƣ�������ˮ�ʼ��������ӷ���ʽΪN2H4��H2ON2H5+��OH-��
(2) ��ԭ�ӵ�2p���Ϊ2p3������ȶ�״̬�������ٵ�������ӣ��ʵ�ԭ�ӵ�һ�����ܴ�����ԭ�ӣ�
(3)N2O��CO2��Ϊ�ȵ����壬����N2O�ĽṹʽΪN=N=O ��
(4) NO2-Ϊ3ԭ�����۵�����Ϊ18����SO2��O3��Ϊ�ȵ����壻
(5)Ti��������Ķѻ�ģ��ΪMg�ͣ��������ܶѻ�����λ��Ϊ12����̬Ti��Χ�����Ų�ʽΪ3d24s2����̬Ti3+��δ�ɶԵ�������1����
(6) ���ݾ�̯��������֪���þ�����Nԭ�Ӹ���Ϊ��6��![]() +8��
+8��![]() =4���þ�����Tiԭ�Ӹ���Ϊ��1+12��
=4���þ�����Tiԭ�Ӹ���Ϊ��1+12��![]() =4����������m=4��
=4����������m=4��![]() g���辧����N��Ti֮����������ΪΪanm��
g���辧����N��Ti֮����������ΪΪanm��
�������V=![]() cm3������
cm3������![]() =
=![]() �ã�m=
�ã�m=![]() V����4��
V����4��![]() g =��g��cm-3��
g =��g��cm-3��![]() cm3�����a=
cm3�����a=![]() ��
��
�Ծ�������Nԭ���о�����֮��������������Tiԭ�ӹ���6����Χ�ɵĿռ伸����Ϊ�������塣
(1)�����֪��������N2H4��Nԭ�ӵ��ӻ���ʽΪsp3����ˮ��Һ�Լ���ԭ����NH3���ƣ�������ˮ�ʼ��Ե����ӷ���ʽΪN2H4��H2ON2H5+��OH-��
�ʴ�Ϊ��sp3��N2H4��H2ON2H5+��OH- ��
(2) ��ԭ�ӵ�2p���Ϊ2p3������ȶ�״̬�������ٵ�������ӣ��ʵ�ԭ�ӵ�һ�����ܴ�����ԭ�ӣ�
�ʴ�Ϊ����ԭ�ӵ�2p���Ϊ2p3������ȶ�״̬�������ٵ�������ӣ��ʵ�ԭ�ӵ�һ�����ܴ�����ԭ�ӣ�
(3)N2O��CO2��Ϊ�ȵ����壬����N2O�ĽṹʽΪN=N=O ��
�ʴ�Ϊ��N=N=O��
(4) NO2-Ϊ3ԭ�����۵�����Ϊ18����SO2��O3��Ϊ�ȵ����壻
p>�ʴ�Ϊ��SO2��O3��(5)Ti��������Ķѻ�ģ��ΪMg�ͣ��������ܶѻ�����λ��Ϊ12����̬Ti��Χ�����Ų�ʽΪ3d24s2����̬Ti3+��δ�ɶԵ�������1����
�ʴ�Ϊ���������ܶѻ���12��1��
(6) ���ݾ�̯��������֪���þ�����Nԭ�Ӹ���Ϊ��6��![]() +8��
+8��![]() =4���þ�����Tiԭ�Ӹ���Ϊ��1+12��
=4���þ�����Tiԭ�Ӹ���Ϊ��1+12��![]() =4����������m=4��
=4����������m=4��![]() g���辧����N��Ti֮����������ΪΪanm��
g���辧����N��Ti֮����������ΪΪanm��
�������V=![]() cm3������
cm3������![]() =
=![]() ����m=
����m=![]() V����4��
V����4��![]() g =��g��cm-3
g =��g��cm-3![]() cm3�����a=
cm3�����a=![]() ��
��
�Ծ�������Nԭ���о�����֮��������������Tiԭ�ӹ���6����Χ�ɵĿռ伸����Ϊ�������塣
�ʴ�Ϊ��![]() ���������塣
���������塣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��(һ)�����£���25 mL 0.1 mol��L-1 CH3COOH��Һ����μ���0.1 mol��L-1NaOH��Һ����������ͼ��ʾ���ش��������⣺

(1)д��CH3COOH�ĵ��뷽��ʽ__________________________��
(2)����˵������ȷ����___________________��
A. 0.1 mol/L CH3COOH��Һ��, CH3COOH�����ԼΪ1%
B. B�����㣺 c(CH3COO-)-c(CH3COOH)=2c(OH-)-2c(H+)
C. C��ʱ������Ũ�ȹ�ϵΪ��c(CH3COO-)= c(Na+)> c(H+)= c(OH-)
D. D��ʱ������Ũ�ȹ�ϵΪ�� c(Na+)> c(CH3COO-)> c(OH-)> c(H+)
(3)�����£��Լ���CH3COOH����ƽ�ⳣ��Ϊ____________���ú�b�ı���ʽ��ʾ����
(��)ʵ����Ϊ�ⶨʳ����CH3COOH��Ũ�ȣ�ȡ25mLʳ������250mL����ƿ�У���ˮϡ�����̶Ȳ�ҡ�ȡ�����ʽ�ζ�����ȡ25.00mLϡ�ͺ�Ĵ�����Һ������ƿ�У���ָʾ����Ȼ����0.1000 mol��L-1NaOH����Һ���еζ���
(4)ָʾ��ӦΪ________��
A.���� B.���� C.��̪ D.ʯ��
(5)�ζ��յ���жϷ���Ϊ____________________________________________________��
(6)Ϊ��߲ⶨ��ȷ�ȣ��ظ�����ʵ�����Σ�0.1000 mol��L-1NaOH����Һ�ζ�ǰ��Ķ������±���ʾ�����ʳ����CH3COOH��Ũ��Ϊ_________mol��L-1��
ʵ����� | ϡ�ͺ�Ĵ�����Һ���/ mL | NaOH�ζ�ǰ����/ mL | NaOH�ζ������/ mL |
��1�� | 25.00 | 0.10 | 24.00 |
��2�� | 25.00 | 0.50 | 22.50 |
��3�� | 25.00 | 0.20 | 24.30 |
(7)��0.1000 mol��L-1NaOH����Һ���еζ������в����ᵼ�²ⶨ���ƫ�ߵ���_______��
A����ʽ�ζ����ڵζ����������
B����ȡ��Һ����ʱ���ζ�ǰ���ӣ��ζ����յ������
C������0.1000 mol��L-1NaOH��Һʱ������NaOH�к��нᾧˮ
D����ʽ�ζ���δ��ϴ��װ���Һ���еζ�
����Ŀ��������������Ӱ�����ǵ�����ͽ�����������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ���˸�����Դ�ṹ���������ŵȴ�ʩ����Ч����PM2.5��SO2��NOx����Ⱦ����ش��������⣺
(1)��һ������ijPM2.5��Ʒ������ˮ�ܽ��Ƴɴ�������(����OH��)�������²�ø���������ɼ���Ũ�����±������ݱ��������жϸ�������pH=___________��
���� | K�� | Na�� | NH4�� | SO42�� | NO3�� | Cl�� |
Ũ��(mol��L-1) | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
(2)����β����NOx��CO�����ɣ�
��֪����������NO�ķ�ӦΪ��N2(g)+O2(g) ![]() 2NO(g) ��H>0�����£������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____��
2NO(g) ��H>0�����£������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____��
A�����������ܶȲ��ٱ仯 B����������ѹǿ���ٱ仯
C��N2��O2��NO�����ʵ���֮��Ϊ1��1��2 D��������ת���ʲ��ٱ仯
(3)Ϊ����SO2���ŷţ���ϴ�Ӻ�SO2�����������п���Ϊϴ�Ӻ�SO2������ϴ�Ӽ��� ___________��
A��NaHCO3������Һ B��FeCl2������Һ C������CaCl2������Һ
(4)����ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���ʹNOx����Ч������Ϊ�����������Ҫ���⡣ij�о���С����ʵ������Ag-ZSM-5Ϊ���������NOת��ΪN2��ת�������¶ȱ仯�����ͼ��ʾ������ʹ��CO���¶ȳ���775K������NO�ķֽ��ʽ��ͣ�����ܵ�ԭ��Ϊ___________________________________����n(NO)/n(CO)=1�������£�Ϊ���õij�ȥNOx���ʣ�Ӧ���Ƶ�����¶���_____K���ҡ�

(5)�����ŷŵĵ������úȼ�ղ����Ķ��������ǵ������������ġ�������ס�������̿�ɴ���������Ⱦ��NO����5L�ܱ������м���NO�ͻ���̿(����������)��һ����������������E��F�����¶ȷֱ���T1�� ��T2��ʱ����ø�����ƽ��ʱ���ʵ���(n/mol)���±���
���� �¶ȡ� | ����̿ | NO | E | F |
��ʼ | 3.000 | 0.100 | 0 | 0 |
T1 | 2.960 | 0.020 | 0.040 | 0.040 |
T2 | 2.975 | 0.050 | 0.025 | 0.025 |
��д��NO�����̿��Ӧ�Ļ�ѧ����ʽ____________________ (E,F���û�ѧʽ��ʾ)��
����T1<T2����÷�Ӧ�Ħ�H___________0��(�>����<����=��)
�ۼ���������ӦT1��ʱ��ƽ�ⳣ��K=__________________��