��Ŀ����
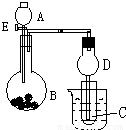
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�a����BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ ��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е����� ��
b����B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���3����ȩ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A�� �������ƣ���C�� ���ѧʽ��������ȩ��Һ��Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ ������D�ڴ�ʵ���е������� ��
���𰸡�������a��̼������Աȱ���ǿ�������Dz�����ˮ�ģ����¶ȵ���16.6��ʱ��Ϊ��ɫ���壬���¶ȸ��ڸ��¶�ʱ�����Ϊ��ɫҺ�壻
B��ȩ�ܺ�������Һ֮�䷢��������Ӧ������������Һ�мӰ�ˮ��ͨ�������������պ���ʧʱ�����Ի��������Һ��
����⣺a��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ�˵���б������ɣ�BΪNa2CO3��ĩ������A��һ��ǿ�ᣬ��̼���Ʒ�Ӧ�ų�������̼��̼������Աȱ���ǿ����������̼ͨ�뵽�������л����ɱ��ӳ�������C6H5O-+H2O+CO2=C6H5OH+HCO3-�����¶ȵ���16.6��ʱΪ��ɫ���壬���ձ��м����ˮ�����ӻ��Ϊ��ɫҺ�壬
�ʴ�Ϊ��C6H5O-+H2O+CO2=C6H5OH+HCO3-�����DZ���壻
B����С�Թ��м���3����ȩ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������Ϊȩ�ܺ�������Һ֮�䷢��������Ӧ������������Һ�мӰ�ˮ��ͨ�������������պ���ʧʱ�����Ի��������Һ������A�ǰ�ˮ��C������������ȩ��������Һ���������ӦΪ��CH3CHO+2[Ag��NH3��2]OH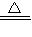 CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO+2[Ag��NH3��2]OH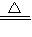 CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O��
������������һ������ʽ����Ŀ��Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�
B��ȩ�ܺ�������Һ֮�䷢��������Ӧ������������Һ�мӰ�ˮ��ͨ�������������պ���ʧʱ�����Ի��������Һ��
����⣺a��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ�˵���б������ɣ�BΪNa2CO3��ĩ������A��һ��ǿ�ᣬ��̼���Ʒ�Ӧ�ų�������̼��̼������Աȱ���ǿ����������̼ͨ�뵽�������л����ɱ��ӳ�������C6H5O-+H2O+CO2=C6H5OH+HCO3-�����¶ȵ���16.6��ʱΪ��ɫ���壬���ձ��м����ˮ�����ӻ��Ϊ��ɫҺ�壬
�ʴ�Ϊ��C6H5O-+H2O+CO2=C6H5OH+HCO3-�����DZ���壻
B����С�Թ��м���3����ȩ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������Ϊȩ�ܺ�������Һ֮�䷢��������Ӧ������������Һ�мӰ�ˮ��ͨ�������������պ���ʧʱ�����Ի��������Һ������A�ǰ�ˮ��C������������ȩ��������Һ���������ӦΪ��CH3CHO+2[Ag��NH3��2]OH
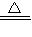 CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O���ʴ�Ϊ��CH3CHO+2[Ag��NH3��2]OH
 CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O��������������һ������ʽ����Ŀ��Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ
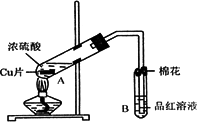
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
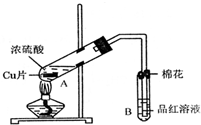
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
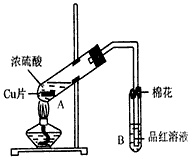
��1��д���Թ�B�е�ʵ������______��
��2��д��A�з�Ӧ�Ļ�ѧ����ʽ______
��3��������A�Թ��м���H2O2������ͭƬ�ܽ⣬��Ӧ�����ӷ���ʽΪ��______��
���Բ�����Ũ���ᣬֻҪ��ʹͭƬ�ܽ⣬Ҳ���Լ��루��д�������ڲ�ͬ������ʵĻ�ѧʽ��______��______��
��4��B�Թܿڵ���Ӧմ�е��Լ���______��
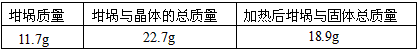
��5��С���Ա��Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4?xH2O����С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ��
�������ǵ�ʵ������У����ٳ���______�Σ�
������������һ��ʵ������ݣ�
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
A������ͭ�����к��в��ӷ������ʡ�������B��ʵ��ǰ���������ʪ��ˮ
C������ʱ�о���ɽ���ȥ����������������D������ʧˮ��¶���ڿ�������ȴ��
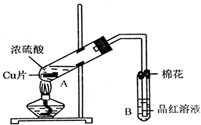 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���