��Ŀ����
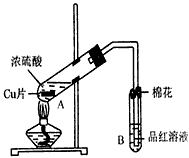
��2��д��A�з�Ӧ�Ļ�ѧ����ʽ______________________��
��3��������A�Թ��м���H2O2������ͭƬ�ܽ⣬��Ӧ�����ӷ���ʽΪ��______________________�����Բ�����Ũ���ᣬֻҪ��ʹͭƬ�ܽ⣬Ҳ���Լ��루��д�������ڲ�ͬ������ʵĻ�ѧʽ��___________��
___________��
��4��B�Թܿڵ���Ӧմ�е��Լ���___________��
��5��С���Ա��Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4��xH2O����С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ��
�������ǵ�ʵ������У����ٳ���___________�Σ�
������������һ��ʵ������ݣ�
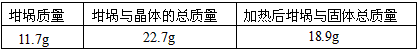
A������ͭ�����к��в��ӷ�������
B��ʵ��ǰ���������ʪ��ˮ
C������ʱ�о���ɽ���ȥ
D������ʧˮ��¶���ڿ�������ȴ
��2��Cu+2H2SO4(Ũ)
 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O��3��Cu+H2O2+2H+==Cu2++2H2O��Fe2O3��NaNO3
��4��NaOH����Һ����KMnO4����Һ��������������Ҳ���ԣ�
��5����4����ƫ��BC

 ��У����ϵ�д�
��У����ϵ�д�ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
��1��д���Թ�B�е�ʵ������ ��
��2��д��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
 ��3����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬������A�м���
��3����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬������A�м���
NaNO3������ͭƬ�ܽ⣬��Ӧ�����ӷ���ʽ
Ϊ ��
��4����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬
�����ְ�ɫ���ǣ��ð�ɫ������ ��
����ȷ�ϸð�ɫ������ʲô���ʵ�ʵ���������
�� ��
��5��B�Թܿڵ���Ӧմ�е��Լ��� ��
��6��С���Ա��4����Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ��ᾧ�Ƶ�����ͭ���壨CuSO4��xH2O����С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ
�������ǵ�ʵ������У����ٳ����ĴΣ�������γ�����Ŀ����
������������һ��ʵ������ݣ�
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.0g | 37.8g | 27.0g |
�����ϱ����ݼ����ж�x��ʵ��ֵ������ֵ��x��5�� ���ƫ����ƫС������

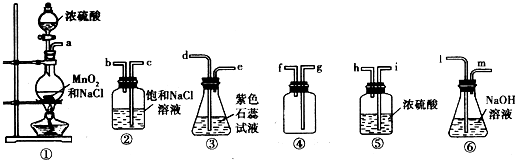
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���