��Ŀ����
����Ŀ��I.����������Һ���ֱ��������ӣ���Ag+����Mg2+����Fe2+����Al3+����Fe3+��
(1)д�������������������ӷ��������ܱ��������ܱ���ԭ��������______�������ۺ���Һ���ص���____��
(2)��Fe2+����Һ�еμ�NaOH��Һ��������__________________��
(3)��ȥFeCl2��FeCl3�����漰�����ӷ���ʽ��________________��
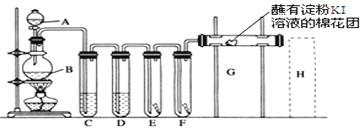
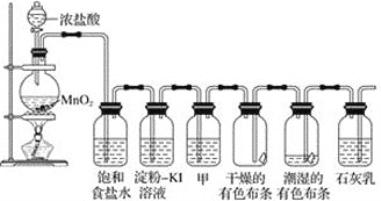
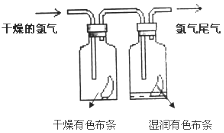
II.(1)��ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ��ʪ����ɫ�����Ĺ��ƿ���ɹ۲쵽�������ǣ�____________����ʵ��֤����Ư�����õ���______��(�ѧʽ)
(2)�������ж���ʵ�������ն����������ԭ����(�����ӷ���ʽ��ʾ)__________________��
�ڸ�����һԭ������ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���_________(�ѧʽ)��
�۳���¶���ڿ����е�Ư�ۻ�ʧЧ��ʧЧ��ԭ����(�û�ѧ����ʽ��ʾ)___________________________��________________________��
��Ư���Ƿ���ȫʧЧ����ϡ������飬��ϡ����������������______(����ĸ����)��
A.O2 B.Cl2 C.CO2 D.HClO
���𰸡�Fe2+ Fe3+ ���ɰ�ɫ��״����������Ѹ���ɰ�ɫ��Ϊ����ɫ������Ϊ���ɫ Fe+2Fe3+�T3Fe2+ �������ɫ��������������ʪ����ɫ������ɫ HClO Cl2+2OH-=Cl-+ClO-+H2O Ca(ClO)2 Ca(ClO)2+CO2+H2O=CaCO3��+2HClO 2HClO![]() 2HCl+O2�� C
2HCl+O2�� C
��������
I. (1)�����м��̬�Ľ��������Ӽ��ܱ��������ܱ���ԭ�����ܽ�������Һ���û��������ܺ�Fe3+��Ӧ����Fe2+��
(2) ��Fe2+����Һ�еμ�NaOH��Һ��������Ӧ��Fe2++2OH-=Fe(OH)2������ɫ���������������������ױ���������Ϊ����������
(3) Fe3+�ܱ�����ԭ����Fe2+���Ҳ�������������
II.(1)������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�
(2)��������������������Һ��Ӧ������β����ʯ�������չ�ҵ����β���Ƶ�Ư�������Ȼ��ơ�������ƣ��������ΪƯ�۵���Ч�ɷ֣�����¶���ڿ����е�Ư�ۣ�������Ʊ���Ϊ̼��ƣ���ϡ��������������Ϊ������̼.
I.(1)Fe2+��FeԪ�صĻ��ϼ۴����м��̬�����ܱ��������ܱ���ԭ��������ٵ�Ag+�͢���Fe3+�������ӷ�Ӧ���Ԣٷ�ӦΪ��Fe+2Ag+=2Ag+Fe2+����Һ����������ڢݷ�����ӦΪ��Fe+2Fe3+=3Fe2+����Һ�����������ʼ��ܱ��������ܱ���ԭ��������Fe2+�������ۺ���Һ���ص���Fe3+��
(2) Fe2+������������Һ�е�OH-��Ӧ���ɵ���������������Fe2++2OH-=Fe(OH)2�������������������ױ���������Ϊ����������4Fe(OH)2+O2+2H2O=4Fe(OH)3�����Կ����������ǣ����ְ�ɫ������Ѹ�ٱ�Ϊ����ɫ����Ϊ���ɫ��
(3)��ȥFeCl2��FeCl3ѡ�����ۣ� FeCl3����Fe��Ӧ����FeCl2��Һ�������������ʣ���Ӧ�����ӷ���ʽΪ��Fe+2Fe3+=3Fe2+��
II.(1)������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�Cl2+H2O=HCl+HClO����˻ῴ�����������ɫ��������������ʪ����ɫ������ɫ����ʵ��֤����Ư�����õ���HClO��
(2) ��Ϊ�˷�ֹ����β����Ⱦ����������NaOH��Һ���գ��÷�Ӧ�����ӷ���ʽΪCl2+2OH--=Cl-+ClO-+H2O��
��Cl2��ʯ���鷢����Ӧ��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O���õ�Ư�ۣ�Ư�۵���Ч�ɷ���Ca(ClO)2��
�۳���¶���ڿ����е�Ư�ۣ���Ч�ɷ�Ca(ClO)2��Ϳ����еĶ�����̼ˮ��Ӧ����̼��ƺʹ����ᣬ����ʽΪ��Ca(ClO)2+CO2+H2O=CaCO3��+2HClO��HClO���ȶ����������ֽ⣬�ֽⷴӦ����ʽΪ��2HClO![]() 2HCl+O2����
2HCl+O2����
��Ư�۳��ڱ�¶�ڿ����У����õ�����Ҫ�ɷ��к�̼��ƣ���ϡ����������ֽⷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2�����ʺ���ѡ����C��