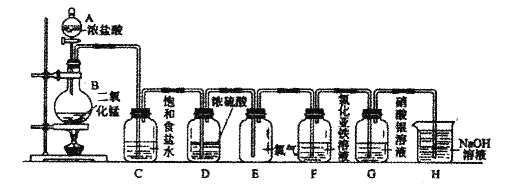
��Ŀ����
����Ŀ�����е����ʵ�����NaHCO3��KHCO3�Ļ����ag��100mL���ᷴӦ��������Ƶ�����������Ա�״���ƣ����ʱ�����ô���ĸ�Ĺ�ʽ��ʾ��
��1���û������NaHCO3��KHCO3��������Ϊ��____��
��2����̼������������ǡ����ȫ��Ӧ�������Ũ��Ϊ��____��
��3�����������������CO2�������____��
��4�������Ӧ��̼��������ʣ�࣬�������Ҫ�������ɵ�CO2�����������֪����____��
��5����NaHCO3��KHCO3�����Ե����ʵ�����ϣ���ag����������������������ȫ��Ӧʱ����CO2�������Χ�ǣ�____��
���𰸡�84��100 ![]() mol/L
mol/L ![]() L ��������ʵ���Ũ��
L ��������ʵ���Ũ�� ![]() L��n��CO2����
L��n��CO2����![]() L
L
��������
��1������m=nM���������ȣ�
��2����NaHCO3��KHCO3�����ʵ�������xmol����![]() ��x=
��x=![]() mol�����ݷ�ӦHCO3-+H+=CO2��+H2O������������ʵ����������������ʵ���Ũ�ȣ�
mol�����ݷ�ӦHCO3-+H+=CO2��+H2O������������ʵ����������������ʵ���Ũ�ȣ�
��3������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3�����ٸ���V=nVm�������ɵĶ�����̼�������
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�������
��5���ٶ�̼������ȫ��NaHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ������ٶ�̼������ȫ��KHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ�����ʵ�����ɶ�����̼�����ʵ����ڶ���֮�䣬����ȷ�������Χ��
��1�������ʵ�����NaHCO3��KHCO3������Ϊ![]() ��
��
��2��NaHCO3��KHCO3�����ʵ�������![]() mol��HCO3-�����ʵ�����
mol��HCO3-�����ʵ�����![]() mol,����HCO3-+H+=CO2��+H2O��n��H+��=
mol,����HCO3-+H+=CO2��+H2O��n��H+��=![]() mol��c��HCl��=
mol��c��HCl��=![]() mol��0.1L=
mol��0.1L=![]() mol/L��
mol/L��
��3�����������NaHCO3��KHCO3��ɵĻ������ȫ��Ӧ������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3��=![]() mol�����Ա�״��������CO2�����Ϊ=
mol�����Ա�״��������CO2�����Ϊ=![]() mol��22.4L/mol=
mol��22.4L/mol=![]() L��
L��
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�����������Ҫ��������CO2�����������Ҫ֪����������ʵ���Ũ�ȣ�
��5���ٶ�̼������ȫ��NaHCO3����n��NaHCO3��=![]() mol;NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO
mol;NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO![]() mol��
mol��
�ٶ�̼������ȫ��KHCO3����n��KHCO3��=![]() mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=
mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=![]() mol������ʵ�ʶ�����̼�����ʵ���Ϊ
mol������ʵ�ʶ�����̼�����ʵ���Ϊ![]() mol��n��CO2����
mol��n��CO2����![]() mol���ʱ������������Χ��
mol���ʱ������������Χ��![]() L��n��CO2����
L��n��CO2����![]() L��
L��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�