��Ŀ����
25 ��ʱ������ƽ�ⳣ����
�ش��������⣺
(1)���ʵ���Ũ��Ϊ0.1 mol��L��1�������������ʣ�a.Na2CO3��b.NaClO��c.CH3COONa��d.NaHCO3��pH�ɴ�С��˳����______________(����)��
(2)������0.1 mol��L��1��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С����________������������������������������������
A��c(H��) B.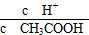 C��c(H��)��c(OH��) D.
C��c(H��)��c(OH��) D.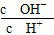
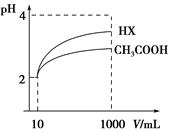
(3)���Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX��Һ�ֱ��ˮϡ����1 000 mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��________(����ڡ��������ڡ���С�ڡ�)�����ƽ�ⳣ����������__________________________________________��ϡ�ͺ�HX��Һ��ˮ���������c(H��)________������Һˮ�������c(H��)(����ڡ��������ڡ���С�ڡ�)�����ǣ�____________________________________��
(4)25 ��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH��6������Һ��c(CH3COO��)��c(Na��)��________(��ȷ��ֵ)��
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10��5 | K1 4.3��10��7 K2 5.6��10��11 | 3.0��10��8 |
�ش��������⣺
(1)���ʵ���Ũ��Ϊ0.1 mol��L��1�������������ʣ�a.Na2CO3��b.NaClO��c.CH3COONa��d.NaHCO3��pH�ɴ�С��˳����______________(����)��
(2)������0.1 mol��L��1��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С����________������������������������������������
A��c(H��) B.
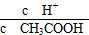 C��c(H��)��c(OH��) D.
C��c(H��)��c(OH��) D.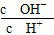
(3)���Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX��Һ�ֱ��ˮϡ����1 000 mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��________(����ڡ��������ڡ���С�ڡ�)�����ƽ�ⳣ����������__________________________________________��ϡ�ͺ�HX��Һ��ˮ���������c(H��)________������Һˮ�������c(H��)(����ڡ��������ڡ���С�ڡ�)�����ǣ�____________________________________��

(4)25 ��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH��6������Һ��c(CH3COO��)��c(Na��)��________(��ȷ��ֵ)��
��(1)a��b��d��c ��(2)A��(3)���ڡ�ϡ����ͬ������HX��Һ��pH�仯��CH3COOH��Һ�Ĵ�����ǿ������ƽ�ⳣ�����ڡ�HX����ǿ��CH3COOH��ϡ�ͺ�c(H��)С��CH3COOH��Һ�е�c(H��)�����Զ�ˮ��������������
(4)9.9��10��7 mol��L��1
(4)9.9��10��7 mol��L��1
��(1)�۲����ƽ�ⳣ����֪����ΪCH3COOH>H2CO3>HClO>HCO3��������Щ��ʧȥ�����Ӻ�ˮ������ȴ�����෴�����Եó�pH��С˳��Ϊa��b��d��c��(2)������������ʣ�ϡ�ͺ����̶�����CH3COOH��CH3COO����H��Ũ��ȴ��Ҫ��С����c(OH��)ȴ������ģ���CH3COOHŨ�ȼ�����࣬���A����ȷ��(3)ϡ����ͬ������HX��Һ��pH�仯��CH3COOH��Һ�Ĵ�����ǿ������ƽ�ⳣ����HX�ĵ��볣������CH3COOH�ĵ��볣����HX����ǿ��CH3COOH��ϡ�ͺ�c(H��)С��CH3COOH��Һ�е�c(H��)�����Զ�ˮ����������������(4)���ݵ���غ��c(CH3COO��)��c(Na��)��c(H��)��c(OH��)��10��6 mol��L��1��10��8 mol��L��1��9.9��10��7 mol��L��1��

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
�����Ŀ
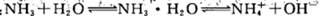 �ı�������������ʹ����̶��������
�ı�������������ʹ����̶��������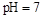 �Ļ����Һ��:c(CH3COO-)+c(CH3COOH)<c(Na+)
�Ļ����Һ��:c(CH3COO-)+c(CH3COOH)<c(Na+) 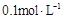 ���������Һ��:c(NH4+)+c(H+)��c(SO42-)+c(OH-)
���������Һ��:c(NH4+)+c(H+)��c(SO42-)+c(OH-) 