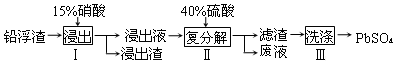
��Ŀ����
15��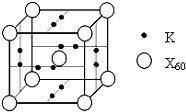 ��֪X��Y��Z��Q��E����Ԫ�ص�ԭ������������������Xԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��s�ܼ���������p�ܼ���������������Zԭ��L������2�ԳɶԵ��ӣ�Q�ǵ��������е縺������Ԫ�أ�E�ĵ����dz�����Ψһ��Һ̬�ķǽ�������ش��������⣺
��֪X��Y��Z��Q��E����Ԫ�ص�ԭ������������������Xԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��s�ܼ���������p�ܼ���������������Zԭ��L������2�ԳɶԵ��ӣ�Q�ǵ��������е縺������Ԫ�أ�E�ĵ����dz�����Ψһ��Һ̬�ķǽ�������ش��������⣺��1��X��Y��Z��һ��������С�����˳��ΪC��O��N����Ԫ�ط��ţ���Y�ļ��⻯��ķ��ӿռ乹���������Σ�������ԭ�Ӳ�ȡsp3�ӻ��������ԣ�����ԡ��Ǽ��ԡ������ӣ�
��2��EԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s24p5��
��3��XZ2�������2�����
��4��Z�⻯��ķе��Q�⻯��ķе�ߣ���������Ԫ�صĵ縺�Ժ�ǿ��ˮ����֮���������������۷е����ߣ�
��5��XԪ�ؿ��γ�X60���ʣ���������ز�����һ��������һ�ָ���ϩ������侧����ͼ��ʾ������λ������������ĺͶ��㣬С����λ������������ϣ����û�������X60���ԭ�Ӹ�����Ϊ1��3��
���� X��Y��Z��Q��E����Ԫ�ص�ԭ������������������Xԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����Xԭ�Ӻ��������Ϊ6����XΪ̼Ԫ�أ�Zԭ��L������2�ԳɶԵ��ӣ���Zԭ�Ӻ�������Ų�Ϊ1s22s22p4����ZΪ��Ԫ�أ�Yԭ����������̼Ԫ������Ԫ��֮�䣬��YΪ��Ԫ�أ�Q�ǵ��������е縺������Ԫ�أ�QΪClԪ�أ�E�ĵ����dz�����Ψһ��Һ̬�ķǽ�����EΪBrԪ�أ�
��1��ͬ����������ҵ�һ�����ܳ��������ƣ�����ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ�ʧȥ������Ҫ�����ϸߣ�
Y�ļ��⻯��ΪNH3��ȷ��Nԭ�Ӽ۲���Ӷ���������ȷ��Nԭ���ӻ���ʽ�����ݿռ�ṹ�ж�������������Ƿ��غϣ�ȷ�����Ӽ������⣻
��2��EΪBrԪ�أ�ԭ�Ӻ��������Ϊ35�����ݺ�������Ų�������д��
��3��CO2������Cԭ�ӳ�2��C=O˫����˫���к���1���Ҽ���1���м���
��4����Ԫ�صĵ縺�Ժ�ǿ��ˮ����֮���������������۷е����ߣ�
��5�����ݾ�̯�����㾧����X60���ԭ�Ӹ������ݴ˽��
��� �⣺X��Y��Z��Q��E����Ԫ�ص�ԭ������������������Xԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����Xԭ�Ӻ��������Ϊ6����XΪ̼Ԫ�أ�Zԭ��L������2�ԳɶԵ��ӣ���Zԭ�Ӻ�������Ų�Ϊ1s22s22p4����ZΪ��Ԫ�أ�Yԭ����������̼Ԫ������Ԫ��֮�䣬��YΪ��Ԫ�أ�Q�ǵ��������е縺������Ԫ�أ�QΪClԪ�أ�E�ĵ����dz�����Ψһ��Һ̬�ķǽ�����EΪBrԪ�أ�
��1��ͬ����������ҵ�һ�����ܳ��������ƣ�����ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ�ʧȥ������Ҫ�����ϸߣ���NԪ�صĵ�һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������C��O��N��
Y�ļ��⻯��ΪNH3��Ϊ�����νṹ��Nԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4��Nԭ���ӻ���ʽΪsp3������������������IJ��غϣ����ڼ��Է��ӣ�
�ʴ�Ϊ��C��O��N�������Σ�sp3��N���ԣ�
��2��EΪBrԪ�أ�ԭ�Ӻ��������Ϊ35����������Ų�ʽΪ��1s22s22p63s23p63d104s24p5���ʴ�Ϊ��1s22s22p63s23p63d104s24p5��
��3��CO2������Cԭ�ӳ�2��C=O˫����˫���к���1���Ҽ���1���м�����CO2�����к���2���м����ʴ�Ϊ��2��
��4����Ԫ�صĵ縺�Ժ�ǿ��ˮ����֮���������������۷е����ߣ���Z�⻯��ķе��Q�⻯��ķе�ߣ�
�ʴ�Ϊ����Ԫ�صĵ縺�Ժ�ǿ��ˮ����֮���������������۷е����ߣ�
��5���ɾ����ṹ��֪��������X60��ĿΪ1+8��$\frac{1}{8}$=2��Kԭ����ĿΪ2��6��$\frac{1}{2}$=6���ʾ�����X60���ԭ�Ӹ���֮��Ϊ2��6=1��3���ʴ�Ϊ��1��3
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����ɡ������ܡ���ѧ�����������������ȣ��Ѷ��еȣ�ע�����þ�̯�����о����ļ��㣮

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�| A�� | NaHCO3��Һ�����CO32-+2 H+=H2O+CO2�� | |
| B�� | ��������Һ��ͭ��Cu+Ag+=Cu2++Ag | |
| C�� | ��������ˮ��Ӧ��K+H2O�TK++OH-+H2�� | |
| D�� | �ô����ˮ����2CH3COOH+CaCO3=Ca2++2CH3COO-+H2O+CO2�� |
| A�� | S��s��+O2��g����SO2��g��+Q1kJ��S��g��+O2��g����SO2��g��+Q2kJ | |
| B�� | H2��g��+$\frac{1}{2}$O2��g����H2O��l��+Q1kJ��H2��g��+$\frac{1}{2}$O2��g����H2O��g��+Q2kJ | |
| C�� | NaOH��aq��+HCl��aq����NaCl��aq��+H2O��l��+Q1kJ NaOH��aq��+HAc��aq����NaAc��aq��+H2O��l��+Q2kJ | |
| D�� | H2��g��+Cl2��g����2HCl��g��+Q1kJ��H2��g��+I2��g����2HI��g��+Q2kJ |

�ش��������⣺
��1����ѧ�����ƱȽϣ��ŵ�֮һ�Dz���ͨ�磮
��2���Ƽ��������ȼ�Һ��ϴ�������dz�ȥ�Ƽ��������ۣ��Ƽ�����ֻ���Ŀ������ǿ��ˮ�Լ�����Ӵ������
��3���Ƽ�����AgNO3��Һ�Ƽ�����������SnCl2��AgNO3��ԭ�����д����ԵĽ���������Ӧ�Ļ�ѧ����ʽ��2SnCl2+4AgNO3�T4Ag+SnCl4+Sn��NO3��4��
��4������ʱ����Һ�е�Ni2+��H2PO2-�ڴ������Ϸ�Ӧ��������ͬʱ������ǿ��H3PO3������������ʵ�����ȵ��������÷�Ӧ�����ӷ���ʽ��2H2O+Ni2++2H2PO2-=Ni++H2��+2H3PO3��
��5����ѧ��ij�ֽ���ʱ����Ӧʱ����Ʋ��ȵ�ʵ�����ݼ�¼�����ʾ��
| ��Ӧʱ��t/s | 1 | 4 | 9 | 16 |
| �Ʋ���y/nm | a | 2a | 3a | 4a |
��6����ѧ������Һ�к���Ni 2+����Ⱦ���ת��Ϊ������ȥ����֪25�棬Ksp[Ni��OH��2]=2.0��10-15����������ʹ��Һ��pH=10��������ķ�Һ����Ԫ�صĺ���Ϊ1.2��10-2mg•L-1��
| W | X | |
| Y | Z |
| A�� | Y����̬�⻯�����ȶ� | B�� | Z�ĵ��������ӻ�ԭ����ǿ | ||
| C�� | X���ʳ����»�ѧ���ʻ��� | D�� | Y��ԭ��������W��7 |
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ksp��Ka | Ksp=1.8��10-10 | Ksp=9.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ����Һ�У���������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ����������Һ��ͨ������CO2�����ӷ���ʽΪ��2ClO-+CO2+H2O=CO32-+2HClO | |
| C�� | ��0.1 mol•L-1CH3COOH��Һ�еμ�NaOH��Һ����c��CH3COOH����c��CH3COO-��=5��9����ʱ��Һ��pH=5 | |
| D�� | ��Ũ�Ⱦ�Ϊ1.0��10-3 mol•L-1��KCl��K2CrO4�����Һ�еμ�1.0��10-3 mol•L-1��AgNO3��Һ��CrO42-���γɳ��� |
| A�� | ��ۺ��������ԣ�X��Y | B�� | X��Y��Z���γ����ӻ����� | ||
| C�� | W���γ�˫ԭ�ӷ��� | D�� | M��W�γɵĻ����ﺬ���Թ��ۼ� |
 ��
��