��Ŀ����
11��������ͼװ�ÿɲⶨm g������Ʒ��������NaCl����Na2CO3������������
��1��ʵ����ʹ�õ�ҩƷ�����У�a��ŨH2SO4��b��ϡ���ᡢc��ϡ���ᡢd��������Ʒ��e����ʯ�ҡ�f��NaOH��Һ��g���������뽫��ЩҩƷ��ʢװ���ֶԺ���������Ҫʱ���ظ�ʹ�ã�������д��Ӧ����ţ�
��g����f����d����b����a��
��e����e��
��2��ʵ����ʹ���˿�������������ʹ��Ӧ�����Ķ�����̼�������ų�����ͨ��������ٶȹ����ͨ������������㣬�����ⶨNa2CO3�ĺ���ƫ�ͣ��ƫ�ߡ�����ƫ�͡���ȷ������
��3������ܢ�������Ƿ�ֹ�����еĶ�����̼��ˮ���������ܢ��У���ȱ������ܢ�������ʹ�������Ʒ��Na2CO3����������ƫ��
��4������Һ©���Тܵĵ�Һ�ٶȹ��죬������ʵ����ƫ�ͣ��ƫ�ߡ�����ƫ�͡��͡�ȷ������
��5��������ܢ���ҩƷ������ʵ��ǰΪm1 g��ʵ���Ϊm2 g������Ʒ��Na2CO3��������������ѧ����ʽΪ$\frac{53��{m}_{2}-{m}_{1}��}{22m}$��100%��
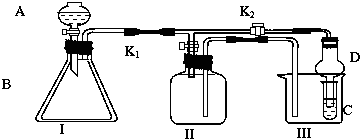
���� ��1������װ��ͼ��֪��ʵ��ԭ��������̼���ƺ��ᷴӦ���ɶ�����̼���������ն�����̼��������̼���Ƶ��������������ɵĶ�����̼�к���ˮ������������ͨ���ʯ��֮ǰ����Ҫ�ȳ�ȥˮ������ͨ���������װ���в����Ķ�����̼����ȫ���ϳ���Ϊ��������ж�����̼Ӱ�죬ͨ��ǰ��Ҫ��ȥ�����еĶ�����̼���ҷ�ֹ������ˮ�����Ͷ�����̼����ʯ�����գ����������ʢ��ʯ�ҵĸ���ܣ�
��2��������֪ʹ�ÿ�����Ŀ���ǰ�װ����ʣ��Ķ�����̼�ų�����ͨ������ٶȹ����ͨ����������������ɵĶ�����̼���ܱ���ȫ������ɽ��ƫ�ͣ�
��3��װ���и���ܢ��Ƿ�ֹ�����е�ˮ�����Ͷ�����̼�������ܢ���û�л�ʹ����ܢ�ⶨ�Ķ�����̼��������
��4������Һ©���Тܵĵ�Һ�ٶȹ���̼���Ʒ�Ӧ����ȫ�����ɶ�����̼�����٣�
��5��������ܢ���ҩƷ������ʵ��ǰΪm1 g��ʵ���Ϊm2 g�������ɶ�����̼����=m2-m1������̼Ԫ���غ����̼��������������
��� �⣺��1������װ��ͼ��֪��ʵ��ԭ��������̼���ƺ��ᷴӦ���ɶ�����̼���������ն�����̼��������̼���Ƶ��������������ɵĶ�����̼�к���ˮ������������ͨ���ʯ��֮ǰ����Ҫ�ȳ�ȥˮ������ͨ���������װ���в����Ķ�����̼����ȫ���ϳ���Ϊ��������ж�����̼Ӱ�죬ͨ��ǰ��Ҫ��ȥ�����еĶ�����̼���ҷ�ֹ������ˮ�����Ͷ�����̼����ʯ�����գ����������ʢ��ʯ�ҵĸ���ܣ�����װ������˳��Ϊ��gfdbaee��
�ʴ�Ϊ��gfdbaee��
��2��ʵ����ʹ�ÿ�����Ŀ���ǰ�װ���в����Ķ�����̼�ų�����ͨ������ٶȹ����ͨ����������������ɵĶ�����̼���ܱ���ȫ������ɽ��ƫ�ͣ�
�ʴ�Ϊ��ʹ��Ӧ�����Ķ�����̼�������ų���ƫ�ͣ�
��3��װ���и���ܢ��Ƿ�ֹ�����е�ˮ�����Ͷ�����̼�������ܢ�Ӱ��ʵ��ⶨ�������û�и���ܢ��ʹ����ܢ�ⶨ�Ķ�����̼��������ʹ�������Ʒ��Na2CO3����������ƫ��
�ʴ�Ϊ����ֹ�����еĶ�����̼��ˮ���������ܢ��У�ʹ�������Ʒ��Na2CO3����������ƫ��
��4������Һ©���Тܵĵ�Һ�ٶȹ���̼���Ʒ�Ӧ����ȫ�����ɶ�����̼�����٣�ʹ�������Ʒ��Na2CO3����������ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��5��������ܢ���ҩƷ������ʵ��ǰΪm1 g��ʵ���Ϊm2 g�������ɶ�����̼����=m2-m1������̼Ԫ���غ����̼������������=$\frac{��{m}_{2}-{m}_{1}��g}{44g/mol}$��106g/mol��100%=$\frac{53��{m}_{2}-{m}_{1}��}{22m}$��100%��
�ʴ�Ϊ��$\frac{53��{m}_{2}-{m}_{1}��}{22m}$��100%��
���� ���⿼�������ʺ����IJⶨ������ʵ����̷����жϣ��������Ͷ��������ǽ���ؼ���ע��ʵ����̵�����Ӧ�ã���Ŀ�Ѷ��еȣ�

| A�� | ֽ�ϲ�����ͨ������ֽ��Ϊ����֧�����ֽ��ά�ϵ��ǻ�������ˮ�ԣ�����������ˮ���̶��� | |
| B�� | �ؽᾧʱ�����ʵ��ܽ��Խ����Һ��ȴ�ٶ�Խ�����õ��ľ������Խ�� | |
| C�� | �Ʊ���������茶�����Ҫ�������������Һ����������������Һ��ϣ��������������ȹ��� | |
| D�� | ������Ľ���Һ�м������Ƽ���Cu��OH��2����Һ�����ɫ |
| A�� | �������ƹ�����ˮ��Ӧ��2O${\;}_{2}^{2-}$+2H2O�T4OH-+O2�� | |
| B�� | ʵ������Fe��OH��3���壺Fe3++3OH-�TFe��OH��3�����壩 | |
| C�� | �����ʯ��ˮ��ͨ�����CO2��OH-+CO2�THCO3- | |
| D�� | ��0.1mol/L��NH4Al��SO4��2��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ�2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� |
| A�� | H+��Na+��S2-��Cl- | B�� | OH-��ClO-��SO42-��S2- | ||
| C�� | H+��MnO4-��Cl-��K+ | D�� | K+��NO3-��Cl-��Fe2+ |
| A�� | SO2�ܽ���ˮ | B�� | NO2�ܽ���ˮ | C�� | CO2�ܽ���ˮ | D�� | Cl2�ܽ���ˮ |
| A�� | ��������NaOH��Һ | B�� | ������������ | ||
| C�� | ���� | D�� | ͨ��CO2���� |
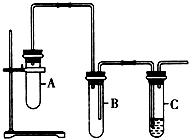 ��֪1��2-�������鳣����Ϊ��ɫҺ�壬�е�83.5�棬�ܶ�1.23g/mL��������ˮ�������ڴ����ѡ���ͪ���л��ܼ����Ҵ��ķе�Ϊ78.5�森ij��ѧ����С��Ϊ̽��1��2-�����������ȥ��Ӧ���������ͼʵ��װ�ã���ش��������⣮
��֪1��2-�������鳣����Ϊ��ɫҺ�壬�е�83.5�棬�ܶ�1.23g/mL��������ˮ�������ڴ����ѡ���ͪ���л��ܼ����Ҵ��ķе�Ϊ78.5�森ij��ѧ����С��Ϊ̽��1��2-�����������ȥ��Ӧ���������ͼʵ��װ�ã���ش��������⣮