题目内容
【题目】铜是重要的金属,广泛应用于电气、机械制造、国防等领域,铜的化合物在科学研究和工农业生产中有许多用途。回答下列问题:
(1)Cu原子的价层电子排布式为________。
(2)CuSO4晶体中S原子的杂化方式为________,SO![]() 的立体构型为________。
的立体构型为________。
(3)向CuSO4溶液中加入过量氨水,可生成[Cu(NH3)4]SO4,下列说法正确的是________。
a.氨气极易溶于水,是因为NH3分子和H2O分子之间形成3种不同的氢键
b.NH3分子和H2O分子,分子立体构型不同,氨气分子的键角小于水分子的键角
c.[Cu(NH3)4]SO4所含有的化学键有离子键、极性共价键和配位键
d.[Cu(NH3)4]SO4的组成元素中电负性最大的是氮元素
【答案】(1)3d104s1 (2)sp3 正四面体 (3)c
【解析】(1)Cu元素的原子序数为29,未成对电子数是1,价层电子排布式为3d104s1;(2)SO![]() 中S原子的价层电子数=
中S原子的价层电子数=![]() =4,孤电子对数为0,采取sp3杂化,立体构型为正四面体;(3)氨气极易溶于水,是由于氨分子中N原子与水分子的H原子形成氢键,a错误;NH3分子和H2O分子的中心原子都是采用sp3杂化,但NH3分子内有1对孤电子对,H2O分子内有2对孤电子对,孤电子对越多对成键电子对的排斥作用力越强,键角被挤压得越小,故氨气分子的键角大于水分子的键角,b错误;[Cu(NH3)4]SO4所含有的化学键有[Cu(NH3)4]2+与SO
=4,孤电子对数为0,采取sp3杂化,立体构型为正四面体;(3)氨气极易溶于水,是由于氨分子中N原子与水分子的H原子形成氢键,a错误;NH3分子和H2O分子的中心原子都是采用sp3杂化,但NH3分子内有1对孤电子对,H2O分子内有2对孤电子对,孤电子对越多对成键电子对的排斥作用力越强,键角被挤压得越小,故氨气分子的键角大于水分子的键角,b错误;[Cu(NH3)4]SO4所含有的化学键有[Cu(NH3)4]2+与SO![]() 之间的离子键、氨分子与Cu2+间的配位键和SO
之间的离子键、氨分子与Cu2+间的配位键和SO![]() 、NH3中的极性共价键,c正确;[Cu(NH3)4]SO4的组成元素中电负性最大的是氧元素,d错误。
、NH3中的极性共价键,c正确;[Cu(NH3)4]SO4的组成元素中电负性最大的是氧元素,d错误。

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案【题目】分别按下图A、B、C所示装置进行实验,图中三个烧杯里的溶液为同浓度的稀硫酸。请回答下列问题:
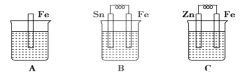
(1)以下叙述中,正确的是__________
A.B中铁片是负极,C中铁片是正极
B.三个烧杯中铁片表面均有气泡产生
C.A、B两烧杯中溶液的pH均增大
D.产生气泡的速率A中比B中慢
E.B溶液中SO42-向Sn电极移动
(2)装置B变化过程中能量转化的形式主要是:____________。
(3)有同学想把Ba(OH)2·8H2O晶体与NH4Cl晶体的反应设计成原电池,你认为是否可行?__________(填“是”或“否”),理由是__________.
(4)A、B、C三个烧杯中铁的腐蚀速率________>_______>______
(5)对于反应2A2+B2=2A2B,已知A2、B2、A2B的键能如下表:
化学键 | A-A | B=B | A-B |
键能KJ/mol | 236 | 406 | 163 |
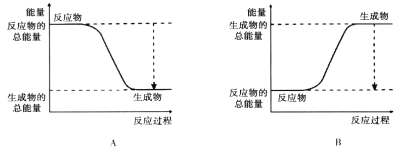
右图能正确表示该反应过程中能量变化的选项是_______。