��Ŀ����
�±���Ԫ�����ڱ���һ���֣�������ĸ�ֱ����ijһ��ѧԪ�أ���ش��й����⡣
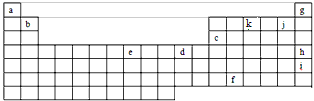
��1����������Ϊg��h��i��������ѧ��Ӧ����������ǽ���ϡ�����壬���Ǻ�����������������Բμӻ�ѧ��Ӧ������Ϊg��h��i�����п������ȱ����ֿ��Բμӻ�ѧ��Ӧ��Ԫ����______________(дԪ�ط���)����ԭ����_________________��
��2��e�����ڱ��е�___________�壬Ԫ��ԭ�ӽṹʾ��ͼΪ_______________��
��3��a�������Ӱ뾶____________b�������Ӱ뾶������ڡ����ڡ�С�ڣ���
��4����Ԫ�����ڱ�����һ���Խ��߹������а���b��c�Ļ����������ʮ�����ơ���b���Ȼ���ֱ�����������������������Һ������Ӧ�����ӷ���ʽ�ֱ�Ϊ��Ҫ����������Ԫ�ط��ţ���ͬ_______________________________��________________________��
��5��f��+4�����������Ũ���ᷴӦ������֪��������ǿ�������������������Ũ���ᷴӦ�Ļ�ѧ����ʽ�ɱ�ʾΪ_________________________��
��6��Ԫ�����ڱ��е�6�����е���ϵԪ�ع���___________�֣�����ԭ�ӵĵ��Ӳ�ṹ������ʮ�����ơ�
��7��Ԫ��k���⻯���ҿ�������10�����ӵ�����ɵĻ�����ף���ѧʽX3Y2����ˮ���ҷ�Ӧ�����ɡ�����ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��________________________��������_______________���壨���ͣ�����ҵ��������������ʱ����Ҫ�����ҵIJ�������Ҫ����ҵIJ��ʣ��ɲ�ȡ�Ĵ�ʩ�ǣ���д���к���ѡ���������_______________��
�ټ�ѹ �ڼ�ѹ ������ �ܽ��� �����˵��¶Ⱥ�ѹǿ �Ӵ���
��ʱ��ƽ����ϵ�з������ �������Һ��ԭ����ѭ��ʹ�ò�����ԭ��
��2��e�����ڱ��е�___________�壬Ԫ��ԭ�ӽṹʾ��ͼΪ_______________��
��3��a�������Ӱ뾶____________b�������Ӱ뾶������ڡ����ڡ�С�ڣ���
��4����Ԫ�����ڱ�����һ���Խ��߹������а���b��c�Ļ����������ʮ�����ơ���b���Ȼ���ֱ�����������������������Һ������Ӧ�����ӷ���ʽ�ֱ�Ϊ��Ҫ����������Ԫ�ط��ţ���ͬ_______________________________��________________________��
��5��f��+4�����������Ũ���ᷴӦ������֪��������ǿ�������������������Ũ���ᷴӦ�Ļ�ѧ����ʽ�ɱ�ʾΪ_________________________��
��6��Ԫ�����ڱ��е�6�����е���ϵԪ�ع���___________�֣�����ԭ�ӵĵ��Ӳ�ṹ������ʮ�����ơ�
��7��Ԫ��k���⻯���ҿ�������10�����ӵ�����ɵĻ�����ף���ѧʽX3Y2����ˮ���ҷ�Ӧ�����ɡ�����ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��________________________��������_______________���壨���ͣ�����ҵ��������������ʱ����Ҫ�����ҵIJ�������Ҫ����ҵIJ��ʣ��ɲ�ȡ�Ĵ�ʩ�ǣ���д���к���ѡ���������_______________��
�ټ�ѹ �ڼ�ѹ ������ �ܽ��� �����˵��¶Ⱥ�ѹǿ �Ӵ���
��ʱ��ƽ����ϵ�з������ �������Һ��ԭ����ѭ��ʹ�ò�����ԭ��
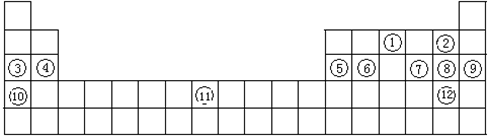
��1��Xe�����ϵ��£�ԭ�Ӱ뾶����ʧȥ����Խ��Խ����
��2��VIII��
��3������
��4��Be2++2OH-=Be(OH)2����Be2++4OH-=BeO22-+2H2O
��5��PbO2+4HCl=PbCl2+Cl2��+2H2O
��6��15
��7��Mg3N2+6H2O=3Mg(OH)2+2NH3�����ӣ��ݢޢߢ�
��2��VIII��

��3������
��4��Be2++2OH-=Be(OH)2����Be2++4OH-=BeO22-+2H2O
��5��PbO2+4HCl=PbCl2+Cl2��+2H2O
��6��15
��7��Mg3N2+6H2O=3Mg(OH)2+2NH3�����ӣ��ݢޢߢ�

��ϰ��ϵ�д�
�����Ŀ
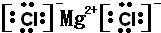
 NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ��
NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ�� ��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����
��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����