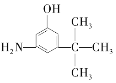
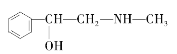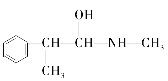��Ŀ����
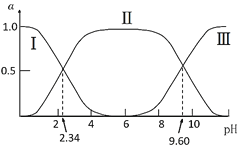
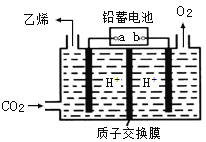
����Ŀ��ˮú���任[CO(g)+H2O(g)��CO2(g)+H2(g) ��H ]����Ҫ�Ļ������̣���Ҫ���ںϳɰ��������Լ��ϳ����ӹ��ȹ�ҵ�����С��ҹ���ѧ������һ�任������˫���ܴ���ͻ���˵����¸�ת������߷�Ӧ���ʲ��ܼ�õ����⡣��Ӧ������ͼ��ʾ��
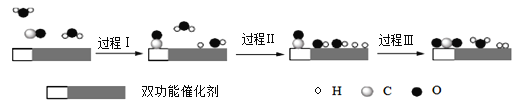
����˵����ȷ����
A.������ʵ�ָ߷�Ӧ��������Ϊʹ����˫���ܴ���������ˮú���任��Ӧ����H
B.���̢�Ϊ���ȹ��̡����̢�Ϊ���ȹ���
C.���̢������˾��зǼ��Թ��ۼ���H2��CO2
D.ͼʾ�е�2��H2O����ֻ��һ��H2O���Ӳ����˷�Ӧ
���𰸡�B
��������
A���������ܸı䷴Ӧ�ġ�H����Aѡ�����
B�����̢���1��ˮ�����еĻ�ѧ�����ѣ�Ϊ���ȹ��̣����̢����γ����µĻ�ѧ����Ϊ���ȹ��̣���Bѡ����ȷ��
C�����̢���CO������ԭ���ź���ԭ���γ��˶�����̼��ˮ��������H2�еĻ�ѧ��Ϊ�Ǽ��Լ���������̼�еĻ�ѧ��Ϊ���Թ��ۼ�����Cѡ�����
D�����̢���1��ˮ�����еĻ�ѧ�����ѣ����̢���һ��ˮ�����еĻ�ѧ�����ѵĹ��̣����̢����γ����µ�ˮ���ӣ������ʼʱ��2��H2O���ն������˷�Ӧ����D����ȷ����
�ʴ�ѡB��

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ