ÌâÄżÄÚÈĘ
ĄŸÌâÄżĄżÄłžß·ÖŚÓ»ŻșÏÎïM”ÄșÏłÉ·ÏßÈçÏÂŁș
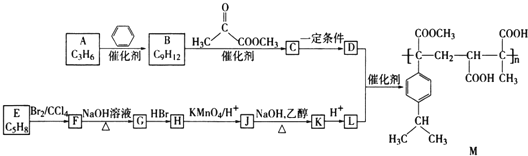
ÒŃÖȘŁșR-CH2OH![]() R-COOH±íÊŸ±„șÍÌț»ù
R-COOH±íÊŸ±„șÍÌț»ù![]() »ŰŽđÏÂÁĐÎÊÌâŁș
»ŰŽđÏÂÁĐÎÊÌâŁș
(1)![]() ”Ä·ŽÓŠÀàĐÍÊÇ ______ Łź
”Ä·ŽÓŠÀàĐÍÊÇ ______ Łź
(2)![]() ”Ä·ŽÓŠÌőŒțÎȘ ______ Łź
”Ä·ŽÓŠÌőŒțÎȘ ______ Łź
(3)![]() żÉÒÔÊčäć”ÄËÄÂÈ»ŻÌŒÈÜÒșÍÊÉ«ŁŹÔòE”ÄÏ”ÍłĂüĂûÊÇ ______ Łź
żÉÒÔÊčäć”ÄËÄÂÈ»ŻÌŒÈÜÒșÍÊÉ«ŁŹÔòE”ÄÏ”ÍłĂüĂûÊÇ ______ Łź
(4)ÉèŒÆ·ŽÓŠ![]() șÍ
șÍ![]() ”ÄÄż”ÄÊÇ ______ Łź
”ÄÄż”ÄÊÇ ______ Łź
(5)![]() ÓĐÁœÖÖżÉÄܔĜáč裏ÎȘÁËÈ·¶šÆäœáččżÉŃĄÓĂ”ÄÒÇÆśÊÇ ______
ÓĐÁœÖÖżÉÄܔĜáč裏ÎȘÁËÈ·¶šÆäœáččżÉŃĄÓĂ”ÄÒÇÆśÊÇ ______ ![]() ÌîŽúșĆ
ÌîŽúșĆ![]() Łź
Łź
![]() șìÍâčâÆŚÒÇ
șìÍâčâÆŚÒÇ![]() ÖÊÆŚÒÇ
ÖÊÆŚÒÇ![]() ÔȘËŰ·ÖÎöÒÇ
ÔȘËŰ·ÖÎöÒÇ![]() șËŽĆčČŐńÒÇ
șËŽĆčČŐńÒÇ
(6)žß·ÖŚÓ»ŻșÏÎïM”Ä”„ÌćÎȘ ______ ![]() ĐŽœáččŒòÊœ
ĐŽœáččŒòÊœ![]() Łź
Łź
(7)![]() ”Ä»ŻŃ§·œłÌÊœÎȘ ______ Łź
”Ä»ŻŃ§·œłÌÊœÎȘ ______ Łź
(8)ÓëLŸßÓĐÏàÍŹčÙÄÜÍĆ”ÄL”ÄÍŹ·ÖÒìččÌć»čÓĐ ______ ÖÖŁŹÆäÖĐșËŽĆčČŐńÇâÆŚÎȘ3Śé·ćŁŹÇÒĂæ»ę±ÈÎȘ3Łș2Łș1”ÄÊÇ ______ ![]() ĐŽœáččŒòÊœ
ĐŽœáččŒòÊœ![]() Łź
Łź
ĄŸŽđ°žĄżŒÓłÉ·ŽÓŠ ĆšÁòË᥹ŒÓÈÈ ![]() ŒŚ»ù
ŒŚ»ù![]() ŁŹ
ŁŹ![]() ¶Ą¶țÏ© ±Ł»€ÌŒÌŒË«ŒüČ»±»Ńő»Ż ad
¶Ą¶țÏ© ±Ł»€ÌŒÌŒË«ŒüČ»±»Ńő»Ż ad 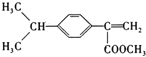
![]()
 +3NaOH
+3NaOH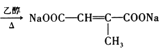 +NaBr+3H2O 4
+NaBr+3H2O 4 ![]()
ĄŸœâÎöĄż
žßŸÛÎïM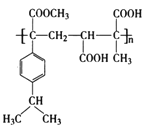 ÓÉDșÍLÔÚŽß»ŻŒÁ”ÄŚśÓĂÏÂÉúłÉŁŹżÉÍÆłöDĄąLÎȘ
ÓÉDșÍLÔÚŽß»ŻŒÁ”ÄŚśÓĂÏÂÉúłÉŁŹżÉÍÆłöDĄąLÎȘ Ąą
Ąą![]() ŁŹ
ŁŹ![]() ŚîÖŐŚȘ»ŻłÉLŁŹËùÒÔLÎȘ
ŚîÖŐŚȘ»ŻłÉLŁŹËùÒÔLÎȘ![]() ŁŹDÎȘ
ŁŹDÎȘ ŁŹAÎȘ
ŁŹAÎȘ![]() șͱœ»·
șͱœ»·![]() ·ŽÓŠÉúłÉ
·ŽÓŠÉúłÉ![]() ÎȘŒÓłÉ·ŽÓŠŁŹÔòAÎȘ
ÎȘŒÓłÉ·ŽÓŠŁŹÔòAÎȘ![]() ŁŹBÎȘ
ŁŹBÎȘ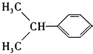 ŁŹBÓë
ŁŹBÓë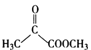 ·ŽÓŠÉúłÉCŁŹCÎȘ
·ŽÓŠÉúłÉCŁŹCÎȘ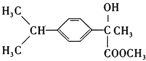 ŁŹC”œDÎȘŽŒ”ÄÏûÈ„ŁŹLÎȘ
ŁŹC”œDÎȘŽŒ”ÄÏûÈ„ŁŹLÎȘ![]() ŁŹKËữ”Ă”œLŁŹKÎȘJÏûÈ„”Ă”œŁŹËùÒÔKÎȘ
ŁŹKËữ”Ă”œLŁŹKÎȘJÏûÈ„”Ă”œŁŹËùÒÔKÎȘ![]() ŁŹžùŸĘ
ŁŹžùŸĘ![]() œáșÏH·ŽÓŠÉúłÉJ
œáșÏH·ŽÓŠÉúłÉJ![]() ŁŹHÎȘ
ŁŹHÎȘ![]() ŁŹŸĘŽËÖđČœÍÆłöŁŹGÎȘ
ŁŹŸĘŽËÖđČœÍÆłöŁŹGÎȘ![]() ŁŹFÎȘ
ŁŹFÎȘ![]() ŁŹEÎȘ
ŁŹEÎȘ![]() ĄŁ
ĄŁ
ąĆ![]() ”Ä·ŽÓŠŁș
”Ä·ŽÓŠŁș![]()
![]()
![]()
 ŁŹÎȘŒÓłÉ·ŽÓŠŁ»čÊŽđ°žÎȘŁșŒÓłÉ·ŽÓŠĄŁ
ŁŹÎȘŒÓłÉ·ŽÓŠŁ»čÊŽđ°žÎȘŁșŒÓłÉ·ŽÓŠĄŁ
ąÆ![]() ÎȘŽŒ”ÄÏûÈ„ŁŹžĂ·ŽÓŠ”Ä·œłÌÊœÎȘŁș
ÎȘŽŒ”ÄÏûÈ„ŁŹžĂ·ŽÓŠ”Ä·œłÌÊœÎȘŁș
![]()

![]() Ł»čÊŽđ°žÎȘŁșĆšÁòË᥹ŒÓÈÈĄŁ
Ł»čÊŽđ°žÎȘŁșĆšÁòË᥹ŒÓÈÈĄŁ
ąÇEÎȘ![]() ŁŹÖśÁŽșŹÓĐ4žöÌŒŁŹ1ŁŹ3șĆÌŒÉÏșŹÓĐË«ŒüŁŹ2șĆÌŒÉÏșŹÓĐŒŚ»ùŁŹËùÒÔÏ”ÍłĂüĂûÊÇ
ŁŹÖśÁŽșŹÓĐ4žöÌŒŁŹ1ŁŹ3șĆÌŒÉÏșŹÓĐË«ŒüŁŹ2șĆÌŒÉÏșŹÓĐŒŚ»ùŁŹËùÒÔÏ”ÍłĂüĂûÊÇ![]() ŒŚ»ù
ŒŚ»ù![]() ŁŹ
ŁŹ![]() ¶Ą¶țÏ©Ł»čÊŽđ°žÎȘŁș
¶Ą¶țÏ©Ł»čÊŽđ°žÎȘŁș![]() ŒŚ»ù
ŒŚ»ù![]() ŁŹ
ŁŹ![]() ¶Ą¶țÏ©ĄŁ
¶Ą¶țÏ©ĄŁ
ąÈ![]() Łș
Łș![]() ŁŹ
ŁŹ![]() Łș
Łș![]()
![]() ŁŹÓÉŽËżÉŒû
ŁŹÓÉŽËżÉŒû![]() șÍ
șÍ![]() ”ÄÄż”ÄÊDZŁ»€ÌŒÌŒË«ŒüČ»±»Ńő»ŻŁ»čÊŽđ°žÎȘŁș±Ł»€ÌŒÌŒË«ŒüČ»±»Ńő»ŻĄŁ
”ÄÄż”ÄÊDZŁ»€ÌŒÌŒË«ŒüČ»±»Ńő»ŻŁ»čÊŽđ°žÎȘŁș±Ł»€ÌŒÌŒË«ŒüČ»±»Ńő»ŻĄŁ
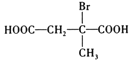
ąÉHÎȘ![]() ŁŹHÓĐÁœÖÖżÉÄܔĜáččŁŹÈ·¶šÆäœáč裏
ŁŹHÓĐÁœÖÖżÉÄܔĜáččŁŹÈ·¶šÆäœáč裏
a.șìÍâčâÆŚÒÇÓĂÓÚŒìČâÓĐ»úÎïÖĐÌŰÊâčÙÄÜÍĆŒ°»úččÌŰŐśŁŹÖśÒȘÊÊÓĂÓÚ¶šĐÔ·ÖÎöÓĐ»ú»ŻșÏÎïœáč裏čÊaŐęÈ·Ł»
b.ÖÊÆŚÒÇÄÜČâłöÓĐ»úÎïÏà¶Ô·ÖŚÓÖÊÁżŁŹÓëœáččÎȚčŰŁŹčÊbŽíÎóŁ»
c.ÔȘËŰ·ÖÎöÒÇÀŽÈ·¶šÓĐ»ú»ŻșÏÎïÖĐ”ÄÔȘËŰŚéłÉŁŹČ»·ûșÏÌâÒ⣏čÊcŽíÎóŁ»
d.șËŽĆčČŐńÒÇÄÜČâłöÓĐ»úÎïÖĐÇâÔŚÓŚÓ”ÄÖÖÀàÒÔŒ°ÊęÄżÖź±ÈŁŹÓëœáččÓĐčŰŁŹčÊdŐęÈ·Ł»
čÊŽđ°žÎȘŁșadĄŁ
ąÊžß·ÖŚÓ»ŻșÏÎïM ŁŹÎȘŒÓŸÛ·ŽÓŠ”ÄČúÎïŁŹËùÒÔDĄąLÎȘ
ŁŹÎȘŒÓŸÛ·ŽÓŠ”ÄČúÎïŁŹËùÒÔDĄąLÎȘ Ąą
Ąą![]() ŁŹ
ŁŹ![]() ŚîÖŐŚȘ»ŻłÉLŁŹËùÒÔLÎȘ
ŚîÖŐŚȘ»ŻłÉLŁŹËùÒÔLÎȘ![]() ŁŹDÎȘ
ŁŹDÎȘ Ł»čÊŽđ°žÎȘŁș
Ł»čÊŽđ°žÎȘŁș Ąą
Ąą![]() Ł»
Ł»
ąË![]() ŁŹÎȘ±ŽúÌț”ÄÏûÈ„ŁŹ·ŽÓŠÎȘŁș
ŁŹÎȘ±ŽúÌț”ÄÏûÈ„ŁŹ·ŽÓŠÎȘŁș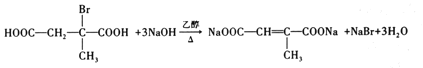 Ł»čÊŽđ°žÎȘŁș
Ł»čÊŽđ°žÎȘŁș +3NaOH
+3NaOH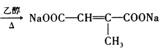 +NaBr+3H2OŁ»
+NaBr+3H2OŁ»
ąÌLÎȘ![]() ŁŹŸßÓĐÏàÍŹčÙÄÜÍĆ”ÄL”ÄÍŹ·ÖÒìččÌć»čÓĐ
ŁŹŸßÓĐÏàÍŹčÙÄÜÍĆ”ÄL”ÄÍŹ·ÖÒìččÌć»čÓĐ![]() Ąą
Ąą![]() Ąą
Ąą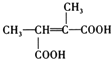 Ąą
Ąą![]() čČ4ÖÖŁŹÆäÖĐșËŽĆčČŐńÇâÆŚÎȘ3Śé·ćŁŹÇÒĂæ»ę±ÈÎȘ3Łș2Łș1”ÄÊÇ
čČ4ÖÖŁŹÆäÖĐșËŽĆčČŐńÇâÆŚÎȘ3Śé·ćŁŹÇÒĂæ»ę±ÈÎȘ3Łș2Łș1”ÄÊÇ![]() Ł»
Ł»
čÊŽđ°žÎȘŁș4Ł»![]() ĄŁ
ĄŁ

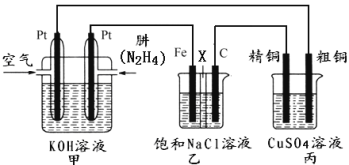
ĄŸÌâÄżĄż![]() ÈÜÒșÓë
ÈÜÒșÓë![]() ÈÜÒșÔÚÈçÍŒËùÊŸ”ÄŚ°ÖĂÖĐœűĐĐÖĐșÍ·ŽÓŠĄŁÍščęČ⶚·ŽÓŠčęłÌÖĐËù·Ćłö”ÄÈÈÁżżÉŒÆËăÖĐșÍÈÈĄŁÇë»ŰŽđÏÂÁĐÎÊÌâ
ÈÜÒșÔÚÈçÍŒËùÊŸ”ÄŚ°ÖĂÖĐœűĐĐÖĐșÍ·ŽÓŠĄŁÍščęČ⶚·ŽÓŠčęłÌÖĐËù·Ćłö”ÄÈÈÁżżÉŒÆËăÖĐșÍÈÈĄŁÇë»ŰŽđÏÂÁĐÎÊÌâ
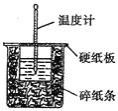
Łš1Ł©ŽÓÊ”Ń錰ÖĂÉÏżŽŁŹÍŒÖĐÉĐȱÉÙ”ÄÒ»ÖÖČŁÁ§ÒÇÆśÊÇ__________________ĄŁ
Łš2Ł©ÉŐ±ŒäÌîÂúËéÖœÌő”ÄŚśÓĂÊÇ__________________ĄŁ
Łš3Ł©Ê”ŃéÊęŸĘÈçϱíŁș
ÎÂ¶È Ê”Ńé ŽÎÊę | ÆđÊŒÎÂ¶È | ÖŐÖčÎÂ¶È | ζÈČîÆœŸùÖ” | ||
| KOH | ÆœŸùÖ” | |||
1 |
|
|
|
| |
2 |
|
|
|
| |
3 |
|
|
|
| |
4 |
|
|
|
| |
![]() ±ŸŽÎÊ”Ńé”ÄζÈČî”ÄÆœŸùÖ”
±ŸŽÎÊ”Ńé”ÄζÈČî”ÄÆœŸùÖ”![]() _________
_________![]() ĄŁ
ĄŁ
![]() œüËÆ”ŰÈÏÎȘ
œüËÆ”ŰÈÏÎȘ![]() ÈÜÒșșÍ
ÈÜÒșșÍ![]() ÈÜÒș”ÄĂܶȶŒÊÇ
ÈÜÒș”ÄĂܶȶŒÊÇ![]() ŁŹÖĐșÍșóÉúłÉÈÜÒș”ıÈÈÈÈĘ
ŁŹÖĐșÍșóÉúłÉÈÜÒș”ıÈÈÈÈĘ![]() ŁŹÔòÖĐșÍÈÈ
ŁŹÔòÖĐșÍÈÈ![]() _________
_________![]() ÊęÖ”Ÿ«È·”œ
ÊęÖ”Ÿ«È·”œ![]() ĄŁ
ĄŁ
![]() ÖĐșÍÈÈČ⶚ʔŃéÖĐŁŹÏÂÁĐČÙŚśÒ»¶š»áœ””ÍÊ”ŃéŚŒÈ·ĐÔ”ÄÊÇ_________ĄŁ
ÖĐșÍÈÈČ⶚ʔŃéÖĐŁŹÏÂÁĐČÙŚśÒ»¶š»áœ””ÍÊ”ŃéŚŒÈ·ĐÔ”ÄÊÇ_________ĄŁ
![]() ÓÔζščÜ
ÓÔζščÜ![]() Ÿ«ÁżÒÇÆśŁŹ¶ÁÊ걣Áô”œ
Ÿ«ÁżÒÇÆśŁŹ¶ÁÊ걣Áô”œ![]() ÈĄËùÓĂËáŒîÈÜÒș”ÄÌć»ę
ÈĄËùÓĂËáŒîÈÜÒș”ÄÌć»ę
![]() ÈÜÒșÔÚ”čÈëĐĄÉŐ±Ê±ŁŹÓĐÉÙÁżœŠłö
ÈÜÒșÔÚ”čÈëĐĄÉŐ±Ê±ŁŹÓĐÉÙÁżœŠłö
![]() ŽóĄąĐĄÉŐ±Ìć»ęÏàČîœÏŽóŁŹŒĐČăŒä·Ć”ÄËéĆĘÄËÜÁϜ϶à
ŽóĄąĐĄÉŐ±Ìć»ęÏàČîœÏŽóŁŹŒĐČăŒä·Ć”ÄËéĆĘÄËÜÁϜ϶à
![]() ČâÁż
ČâÁż![]() ÈÜÒș”ÄζȌÆÓĂËźÏŽŸ»șóČĆÓĂÀŽČâKOHÈÜÒș”ÄζÈ
ÈÜÒș”ÄζȌÆÓĂËźÏŽŸ»șóČĆÓĂÀŽČâKOHÈÜÒș”ÄζÈ
Łš4Ł©Ê”ŃéÖĐžÄÓĂ![]() ŃÎËážú
ŃÎËážú![]() ÈÜÒșœűĐĐ·ŽÓŠŁŹÓëÉÏÊöÊ”ŃéÏà±ÈŁŹËù·Ćłö”ÄÈÈÁż_________
ÈÜÒșœűĐĐ·ŽÓŠŁŹÓëÉÏÊöÊ”ŃéÏà±ÈŁŹËù·Ćłö”ÄÈÈÁż_________![]() ÌÏà”ÈĄ±»òĄ°Č»Ïà”ÈĄ±
ÌÏà”ÈĄ±»òĄ°Č»Ïà”ÈĄ±![]() ŁŹËùÇóÖĐșÍÈÈ_________
ŁŹËùÇóÖĐșÍÈÈ_________![]() ÌÏà”ÈĄ±»òĄ°Č»Ïà”ÈĄ±
ÌÏà”ÈĄ±»òĄ°Č»Ïà”ÈĄ±![]() ĄŁ
ĄŁ
Łš5Ł©ÈôÓĂ![]() ÈÜÒșœűĐĐÉÏÊöÊ”Ń飏Čâ”Ă”ÄÖĐșÍÈÈ”ÄÊęÖ”»á_________
ÈÜÒșœűĐĐÉÏÊöÊ”Ń飏Čâ”Ă”ÄÖĐșÍÈÈ”ÄÊęÖ”»á_________![]() ÌîĄ°Æ«ŽóĄ±ĄąĄ°Æ«ĐĄĄ±»òĄ°ÎȚÓ°ÏìĄ±
ÌîĄ°Æ«ŽóĄ±ĄąĄ°Æ«ĐĄĄ±»òĄ°ÎȚÓ°ÏìĄ±![]() ĄŁ
ĄŁ