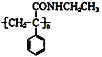
ЬтФПФкШн
ЁОЬтФПЁП![]() ШмвКгы
ШмвКгы![]() ШмвКдкШчЭМЫљЪОЕФзАжУжаНјаажаКЭЗДгІЁЃЭЈЙ§ВтЖЈЗДгІЙ§ГЬжаЫљЗХГіЕФШШСППЩМЦЫужаКЭШШЁЃЧыЛиД№ЯТСаЮЪЬт
ШмвКдкШчЭМЫљЪОЕФзАжУжаНјаажаКЭЗДгІЁЃЭЈЙ§ВтЖЈЗДгІЙ§ГЬжаЫљЗХГіЕФШШСППЩМЦЫужаКЭШШЁЃЧыЛиД№ЯТСаЮЪЬт
ЃЈ1ЃЉДгЪЕбщзАжУЩЯПДЃЌЭМжаЩаШБЩйЕФвЛжжВЃСЇвЧЦїЪЧ__________________ЁЃ
ЃЈ2ЃЉЩеБМфЬюТњЫщжНЬѕЕФзїгУЪЧ__________________ЁЃ
ЃЈ3ЃЉЪЕбщЪ§ОнШчЯТБэЃК
ЮТЖШ ЪЕбщ ДЮЪ§ | Ц№ЪМЮТЖШ | жежЙЮТЖШ | ЮТЖШВюЦНОљжЕ | ||
| KOH | ЦНОљжЕ | |||
1 |
|
|
|
| |
2 |
|
|
|
| |
3 |
|
|
|
| |
4 |
|
|
|
| |
![]() БОДЮЪЕбщЕФЮТЖШВюЕФЦНОљжЕ
БОДЮЪЕбщЕФЮТЖШВюЕФЦНОљжЕ![]() _________
_________![]() ЁЃ
ЁЃ
![]() НќЫЦЕиШЯЮЊ
НќЫЦЕиШЯЮЊ![]() ШмвККЭ
ШмвККЭ![]() ШмвКЕФУмЖШЖМЪЧ
ШмвКЕФУмЖШЖМЪЧ![]() ЃЌжаКЭКѓЩњГЩШмвКЕФБШШШШн
ЃЌжаКЭКѓЩњГЩШмвКЕФБШШШШн![]() ЃЌдђжаКЭШШ
ЃЌдђжаКЭШШ![]() _________
_________![]() Ъ§жЕОЋШЗЕН
Ъ§жЕОЋШЗЕН![]() ЁЃ
ЁЃ
![]() жаКЭШШВтЖЈЪЕбщжаЃЌЯТСаВйзївЛЖЈЛсНЕЕЭЪЕбщзМШЗадЕФЪЧ_________ЁЃ
жаКЭШШВтЖЈЪЕбщжаЃЌЯТСаВйзївЛЖЈЛсНЕЕЭЪЕбщзМШЗадЕФЪЧ_________ЁЃ
![]() гУЕЮЖЈЙм
гУЕЮЖЈЙм![]() ОЋСПвЧЦїЃЌЖСЪ§БЃСєЕН
ОЋСПвЧЦїЃЌЖСЪ§БЃСєЕН![]() ШЁЫљгУЫсМюШмвКЕФЬхЛ§
ШЁЫљгУЫсМюШмвКЕФЬхЛ§
![]() ШмвКдкЕЙШыаЁЩеБЪБЃЌгаЩйСПНІГі
ШмвКдкЕЙШыаЁЩеБЪБЃЌгаЩйСПНІГі
![]() ДѓЁЂаЁЩеБЬхЛ§ЯрВюНЯДѓЃЌМаВуМфЗХЕФЫщХнФЫмСЯНЯЖр
ДѓЁЂаЁЩеБЬхЛ§ЯрВюНЯДѓЃЌМаВуМфЗХЕФЫщХнФЫмСЯНЯЖр
![]() ВтСП
ВтСП![]() ШмвКЕФЮТЖШМЦгУЫЎЯДОЛКѓВХгУРДВтKOHШмвКЕФЮТЖШ
ШмвКЕФЮТЖШМЦгУЫЎЯДОЛКѓВХгУРДВтKOHШмвКЕФЮТЖШ
ЃЈ4ЃЉЪЕбщжаИФгУ![]() бЮЫсИњ
бЮЫсИњ![]() ШмвКНјааЗДгІЃЌгыЩЯЪіЪЕбщЯрБШЃЌЫљЗХГіЕФШШСП_________
ШмвКНјааЗДгІЃЌгыЩЯЪіЪЕбщЯрБШЃЌЫљЗХГіЕФШШСП_________![]() ЬюЁАЯрЕШЁБЛђЁАВЛЯрЕШЁБ
ЬюЁАЯрЕШЁБЛђЁАВЛЯрЕШЁБ![]() ЃЌЫљЧѓжаКЭШШ_________
ЃЌЫљЧѓжаКЭШШ_________![]() ЬюЁАЯрЕШЁБЛђЁАВЛЯрЕШЁБ
ЬюЁАЯрЕШЁБЛђЁАВЛЯрЕШЁБ![]() ЁЃ
ЁЃ
ЃЈ5ЃЉШєгУ![]() ШмвКНјааЩЯЪіЪЕбщЃЌВтЕУЕФжаКЭШШЕФЪ§жЕЛс_________
ШмвКНјааЩЯЪіЪЕбщЃЌВтЕУЕФжаКЭШШЕФЪ§жЕЛс_________![]() ЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАЮогАЯьЁБ
ЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАЮогАЯьЁБ![]() ЁЃ
ЁЃ
ЁОД№АИЁПЛЗаЮВЃСЇНСАшАє![]() ЛђЛЗаЮВЃСЇНСАшЦї МѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇЃЌНЕЕЭЪЕбщЮѓВю 3.4 -56.8kJ/mol
ЛђЛЗаЮВЃСЇНСАшЦї МѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇЃЌНЕЕЭЪЕбщЮѓВю 3.4 -56.8kJ/mol ![]() ВЛЯрЕШ ЯрЕШ ЦЋаЁ
ВЛЯрЕШ ЯрЕШ ЦЋаЁ
ЁОНтЮіЁП
ЃЈ1ЃЉШБЩйвЧЦїЪЧЛЗаЮВЃСЇНСАшАєЃЛ
ЃЈ2ЃЉИУЪЕбщжаашвЊзіКУБЃЮТДыЪЉЃЛ
ЃЈ3ЃЉЂйВюОрНЯДѓЕФЪ§ОнЩсЦњЃЌШЛКѓЧѓЦНОљжЕЃЛ
ЂкИљОнЙЋЪНQ=cmЁїtНјааМЦЫуЃЛ
ЃЈ4ЃЉЗДгІЗХГіЕФШШСПКЭЫљгУЫсвдМАМюЕФСПЕФЖрЩйгаЙиЃЛ
ЃЈ5ЃЉЧтбѕЛЏМиВЛЙ§СПЃЌЗДгІВЛЭъШЋЃЛ
![]() гЩСПШШМЦЕФЙЙдьПЩжЊИУзАжУЕФШБЩйвЧЦїЪЧЛЗаЮВЃСЇНСАшАєЃЛ
гЩСПШШМЦЕФЙЙдьПЩжЊИУзАжУЕФШБЩйвЧЦїЪЧЛЗаЮВЃСЇНСАшАєЃЛ
ЙЪД№АИЮЊЃКЛЗаЮВЃСЇНСАшАєЃЛ
![]() жаКЭШШВтЖЈЪЕбщГЩАмЕФЙиМќЪЧБЃЮТЙЄзїЃЌДѓаЁЩеБжЎМфЬюТњЫщжНЬѕЕФзїгУЪЧМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇЃЌНЕЕЭЪЕбщЮѓВюЃЌ
жаКЭШШВтЖЈЪЕбщГЩАмЕФЙиМќЪЧБЃЮТЙЄзїЃЌДѓаЁЩеБжЎМфЬюТњЫщжНЬѕЕФзїгУЪЧМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇЃЌНЕЕЭЪЕбщЮѓВюЃЌ
ЙЪД№АИЮЊЃКМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇЃЌНЕЕЭЪЕбщЮѓВюЃЛ
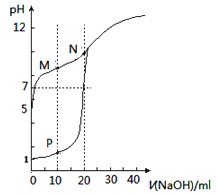
![]() ДЮЮТЖШВюЗжБ№ЮЊЃК
ДЮЮТЖШВюЗжБ№ЮЊЃК![]() ЃЌ
ЃЌ![]() ЃЌ
ЃЌ![]() ЃЌ
ЃЌ![]() ЃЌЕк2зщЪ§ОнЯрВюНЯДѓЃЌЦфЫћШ§ДЮЮТЖШВюЦНОљжЕЮЊЃК
ЃЌЕк2зщЪ§ОнЯрВюНЯДѓЃЌЦфЫћШ§ДЮЮТЖШВюЦНОљжЕЮЊЃК![]() ЃЌ
ЃЌ
ЙЪД№АИЮЊЃК![]() ЃЛ
ЃЛ
![]() ЧтбѕЛЏМигы
ЧтбѕЛЏМигы![]() СђЫсШмвКНјаажаКЭЗДгІЃЌЩњГЩЫЎЕФЮяжЪЕФСПЮЊ
СђЫсШмвКНјаажаКЭЗДгІЃЌЩњГЩЫЎЕФЮяжЪЕФСПЮЊ![]() ЃЌШмвКЕФжЪСПЮЊ
ЃЌШмвКЕФжЪСПЮЊ![]() ЃЌЮТЖШБфЛЏЕФжЕЮЊ
ЃЌЮТЖШБфЛЏЕФжЕЮЊ![]() ЃЌдђЩњГЩ
ЃЌдђЩњГЩ![]() ЫЎЗХГіЕФШШСПЮЊЃК
ЫЎЗХГіЕФШШСПЮЊЃК![]() ЃЌМД
ЃЌМД![]() ЃЌЫљвдЪЕбщВтЕУЕФжаКЭШШ
ЃЌЫљвдЪЕбщВтЕУЕФжаКЭШШ![]() ЃЌ
ЃЌ
ЙЪД№АИЮЊЃК![]() ЃЛ
ЃЛ
![]() гУЕЮЖЈЙм
гУЕЮЖЈЙм![]() ОЋСПвЧЦїЃЌЖСЪ§БЃСєЕН
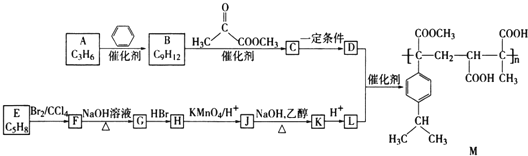
ОЋСПвЧЦїЃЌЖСЪ§БЃСєЕН![]() ШЁЫљгУЫсМюШмвКЕФЬхЛ§ЃЌНсЙћИќОЋШЗЃЌЬсИпЪЕбщзМШЗадЃЌЙЪAДэЮѓЃЛ
ШЁЫљгУЫсМюШмвКЕФЬхЛ§ЃЌНсЙћИќОЋШЗЃЌЬсИпЪЕбщзМШЗадЃЌЙЪAДэЮѓЃЛ
B. ЧтбѕЛЏФЦШмвКНІГіЪБЃЌМюЕФСПМѕЩйЃЌЛсЪЙЕУжаКЭШШЪ§ОнЦЋаЁЃЌетбљвЛЖЈЛсНЕЕЭЪЕбщзМШЗадЃЌЙЪBе§ШЗЃЛ
C. ДѓЁЂаЁЩеБМаВуМфЗХЕФЫщХнФЫмСЯНЯЖрЃЌБЃЮТаЇЙћИќКУЃЌЛсЬсИпЪЕбщзМШЗадЃЌЙЪCДэЮѓЃЛ
D. ВтСПHClШмвКЕФЮТЖШМЦгУЫЎЯДОЛдйВтЧтбѕЛЏФЦЃЌЛсМѕЩйЫсКЭМюжЎМфвђЮЊжаКЭЗДгІЖјЕМжТЕФШШСПЫ№ЪЇЃЌЬсИпЪЕбщЕФзМШЗЖШЃЌЙЪDДэЮѓЁЃ
ЙЪД№АИЮЊЃКBЁЃ
![]() ЗДгІЗХГіЕФШШСПКЭЫљгУЫсвдМАМюЕФСПЕФЖрЩйгаЙиЃЌИФгУ
ЗДгІЗХГіЕФШШСПКЭЫљгУЫсвдМАМюЕФСПЕФЖрЩйгаЙиЃЌИФгУ![]() бЮЫсИњ
бЮЫсИњ![]() ЧтбѕЛЏФЦНјааЗДгІЃЌгыЩЯЪіЪЕбщЯрБШЃЌЩњГЩЫЎЕФСПдіЖрЃЌЫљЗХГіЕФШШСПЦЋДѓЃЌЕЋЪЧжаКЭШШЕФОљЪЧЧПЫсКЭЧПМюЗДгІЩњГЩ1molЫЎЪБЗХГіЕФШШЃЌгыЫсМюЕФгУСПЮоЙиЃЌЫљвдИФгУ
ЧтбѕЛЏФЦНјааЗДгІЃЌгыЩЯЪіЪЕбщЯрБШЃЌЩњГЩЫЎЕФСПдіЖрЃЌЫљЗХГіЕФШШСПЦЋДѓЃЌЕЋЪЧжаКЭШШЕФОљЪЧЧПЫсКЭЧПМюЗДгІЩњГЩ1molЫЎЪБЗХГіЕФШШЃЌгыЫсМюЕФгУСПЮоЙиЃЌЫљвдИФгУ![]() бЮЫсИњ
бЮЫсИњ![]() ЧтбѕЛЏФЦНјааЗДгІЃЌВтЕУжаКЭШШЪ§жЕЯрЕШЃЌ
ЧтбѕЛЏФЦНјааЗДгІЃЌВтЕУжаКЭШШЪ§жЕЯрЕШЃЌ
ЙЪД№АИЮЊЃКВЛЯрЕШЃЛЯрЕШЃЛ
![]() ШєгУ
ШєгУ![]() ШмвКНјааЩЯЪіЪЕбщЃЌЧтбѕИљРызггыЧтРызгЕФЮяжЪЕФСПЯрЕШЃЌЧтбѕЛЏМиВЛЙ§СПЃЌЗДгІВЛЭъШЋЃЌЗХГіЕФШШСПЦЋаЁЃЌВтЕУжаКЭШШЕФЪ§жЕЛсЦЋаЁЃЌ
ШмвКНјааЩЯЪіЪЕбщЃЌЧтбѕИљРызггыЧтРызгЕФЮяжЪЕФСПЯрЕШЃЌЧтбѕЛЏМиВЛЙ§СПЃЌЗДгІВЛЭъШЋЃЌЗХГіЕФШШСПЦЋаЁЃЌВтЕУжаКЭШШЕФЪ§жЕЛсЦЋаЁЃЌ
ЙЪД№АИЮЊЃКЦЋаЁЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
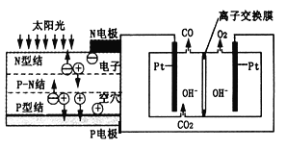
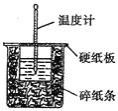
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИЁОЬтФПЁПЙшдкЕиПЧжаЕФКЌСПНЯИпЁЃЙшМАЦфЛЏКЯЮяЕФПЊЗЂгЩРДвбОУЃЌдкЯжДњЩњЛюжагаЙуЗКгІгУЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉ1810ФъШ№ЕфЛЏбЇМвБДВЩРћЮкЫЙдкМгШШЪЏгЂЩАЁЂФОЬПКЭЬњЪБЃЌЕУЕНвЛжжЁАН№ЪєЁБЁЃетжжЁАН№ЪєЁБПЩФмЪЧ_______ЁЃ
ЃЈ2ЃЉЬеДЩЁЂЫЎФрКЭВЃСЇЪЧГЃгУЕФЙшЫсбЮВФСЯЁЃЦфжаЃЌЩњВњЦеЭЈВЃСЇЕФжївЊдСЯга_______ЁЃ
ЃЈ3ЃЉИпДПЙшЪЧЯжДњаХЯЂЁЂАыЕМЬхКЭЙтЗќЗЂЕчЕШВњвЕЖМашвЊЕФЛљДЁВФСЯЁЃЙЄвЕЩЯЬсДПЙшгаЖржжТЗЯпЃЌЦфжавЛжжЙЄвеСїГЬЪОвтЭММАжївЊЗДгІШчЯТЃК
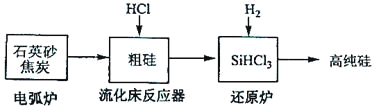
ЗЂЩњЕФжївЊЗДгІ | |
ЕчЛЁТЏ | SiO2+2C |
СїЛЏДВЗДгІЦї | Si+3HCl |
ЛЙдТЏ | SiHCl3+H2 |
ЂйгУЪЏгЂЩАКЭНЙЬПдкЕчЛЁТЏжаИпЮТМгШШвВПЩвдЩњВњЬМЛЏЙшЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______ЃЛЬМЛЏЙшгжГЦ_______ЃЌЦфОЇЬхНсЙЙгы_______ЯрЫЦЁЃ
ЂкдкСїЛЏДВЗДгІЕФВњЮяжаЃЌSiHCl3ДѓдМеМ85%ЃЌЛЙгаSiCl4ЁЂSiH2Cl2ЁЂSiH3ClЕШЃЌгаЙиЮяжЪЕФЗаЕуЪ§ОнШчЯТБэЃЌЬсДПSiHCl3ЕФжївЊЙЄвеВйзївРДЮЪЧГСНЕЁЂРфФ§КЭ_______ЁЃ
ЮяжЪ | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
ЗаЕу/Ёц | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
ЂлSiHCl3МЋвзЫЎНтЃЌЦфЭъШЋЫЎНтЕФВњЮяЮЊ_______ЁЃ
ЃЈ4ЃЉТШМюЙЄвЕПЩЮЊЩЯЪіЙЄвеЩњВњЬсЙЉВПЗждСЯЃЌетаЉдСЯЪЧ_______ЁЃ