��Ŀ����
11��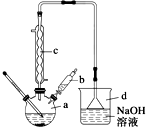 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�����1����a�м���15mL����������м���ٽ�b��4.0mLҺ���������뵽a�У���ַ�Ӧ��
����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mL ˮϴ�ӣ���Һ�ô��屽��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����˼��ôֲ�Ʒ��
| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
��2����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
��3������c��������������������������Ҫ������C6H6��Br2 ���ѧʽ����
��4������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ���������������
���� ��1����������d�����ջӷ�����HBr��
��2�����ݸ÷�ӦΪ���ȷ�Ӧ��������ķе�ϵ����ӷ����з�����
��3�����ݱ������ݿ�֪������Һ��ķе�ϵͣ����ױ������ӷ�������
��4��������Ĵ��屽�к���δ��Ӧ�ı������뻥�ܵ�Һ�壬���ݷе㲻ͬ����������ķ������з��룮
��� �⣺��1������d������Ϊ��©����������ʹHBr��NaOH��Һ��ֽӴ����ʴ�Ϊ������HBr����Ⱦ��
��2������Һ��ķ�ӦΪ���ȷ�Ӧ��������Һ���ٶȹ��죬��Ӧ��ų��϶�����������ڱ�����ķе�ϵͣ����±������ӷ�������Ӱ�����屽�IJ��ʣ�
�ʴ�Ϊ����ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
��3�����ķе�Ϊ80�棬��ķе�Ϊ59�棬���߷е�ϵͣ����ӷ�������������������������Ҫ����Ϊ��C6H6��Br2��
�ʴ�Ϊ��C6H6��Br2��
��4�����÷е㲻ͬ�����ķе�С������������屽����ĸҺ�У����Բ�ȡ����ķ��������屽�뱽���ʴ�Ϊ������
���� ���⿼���˱������ʡ��Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��Դ��漰���屽����ȡʵ�顢���ʵķ����ᴿ�ȣ�����Ʊ���ԭ���ǽ��Ĺؼ������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | $\frac{12W}{n}$ | B�� | $\frac{12n}{W}$ | C�� | 6.02��1023W | D�� | 12��6.02��1023W |
| A�� | SO2��CaO��CO������������ | |
| B�� | ���ᱵ��ˮ����������� | |
| C�� | ����е�1mol•L-1 NaOH��Һ�еμӱ���FeCl3��Һ�Ʊ�Fe��OH��3���� | |
| D�� | �������֡�ˮ��������ˮ��Ϊ����� |
| A�� | ETFE�Ľṹ�У�����-CF2-CH2-CF2-CH2-���ӷ�ʽ | |
| B�� | �ķ���ϩ����ϩ�������۷�Ӧ�õ�ETFE | |
| C�� | ����ϩΪԭ�Ͼ����ӳɷ�Ӧ��ȡ����Ӧ���Ƶ��Ҷ��� | |
| D�� | ETFE�������ȹ��ͣ����νṹ�������� |
| A�� | ��ƽ��ʱ��A2������Ӧ������B2���淴Ӧ������� | |
| B�� | b��0.2mol/L | |
| C�� | �����������䣬�ڷ�Ӧ������ʹ���˴���������� A2��B2��ת���� | |
| D�� | �÷�Ӧ��ƽ��ʱ�������ʵ���Ũ��֮��С�ڣ�a+b+0.3��mol/L |
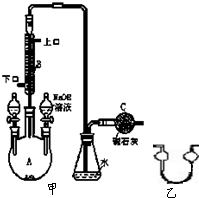 �Ʊ��屽��ʵ��װ����ͼ��ʾ���ش��������⣺
�Ʊ��屽��ʵ��װ����ͼ��ʾ���ش��������⣺ �ǻ���ʯ��һ����Ҫ�����������ϣ��䳣�õ��Ʊ����������֣�
�ǻ���ʯ��һ����Ҫ�����������ϣ��䳣�õ��Ʊ����������֣�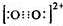 ��CaC2�����ṹ���Ȼ��ƾ������ƣ����ھ�����������Ӿ��������C22-��ĿΪ6����ЩC22-�ڿռ���Χ�ɵļ���ͼ��Ϊ��������
��CaC2�����ṹ���Ȼ��ƾ������ƣ����ھ�����������Ӿ��������C22-��ĿΪ6����ЩC22-�ڿռ���Χ�ɵļ���ͼ��Ϊ��������