��Ŀ����
��ͼ��ʾ���Ǻ����Ƽ��������ʾ��ͼ��
��ش�
(1)ĸҺ�������ܽ����Ҫ������________(д���ʵĻ�ѧʽ)��ĸҺ���к��е�Ũ�ȵ�Na2CO3����Ҫԭ����__________________________
(2)��ĸҺ����ͨ������ʱҪ��ͨ������ͨ������̼���壬��Ҫԭ����_______________________________________
(3)ĸҺ���ĸҺ���ж�ͨ��NH3����NH3����Ҫ��Դ��__________����ĸҺ����ͨ��NH3����ҪĿ����____________����ĸҺ����ͨ��NH3����ҪĿ����__________________��
(4)�������̵ġ����ա�������������____________��ѭ����
(1)NaCl��NaHCO3��NH4Cl��HCO3�������CO32��
(2)NH3��NaCl��Һ���ܽ�Ⱥܴ�CO2�ڰ�������Һ���ܽ�ȴ���ͨNH3��ͨCO2���γɸ�Ũ�ȵ�HCO3��������ͨCO2��ͨNH3������CO2��NaCl��Һ���ܽ�Ⱥ�С������NH3��̼�ữ����Һ���ܽ�ȴ�Ҳ�����γɸ�Ũ�ȵ�HCO3��
(3)�ٺϳɰ�����������NH4����Ũ�ȣ��ٽ�NH4Cl�ᾧ������������OH����Ũ�ȣ��ٽ�CO2���ܽ⣬����HCO3����Ũ��
(4)������̼(��CO2)
����

 �Ķ��쳵ϵ�д�
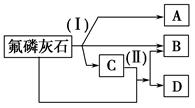
�Ķ��쳵ϵ�д�CaCO3��һ�ֻ���ԭ�ϣ�����ͨ����Ӧ����һϵ�����ʣ�����ͼ��ʾ��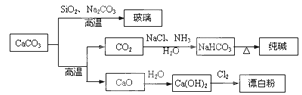
����˵����ȷ����
| A��Cl2��SO2������Ư����ɫ���ʣ���Ư��ԭ����ͬ |
B����SiO2��Na2CO3 Na2SiO3��CO2����֪��H2CO3������ǿ��H2SiO3 Na2SiO3��CO2����֪��H2CO3������ǿ��H2SiO3 |
| C����ҵ�ϣ�������ʳ��ˮ��ͨ��NH3����ͨ��CO2��NaHCO3 |
| D����ȡ�����������Ư�����漰�ķ�Ӧ���Ƿ�������ԭ��Ӧ |
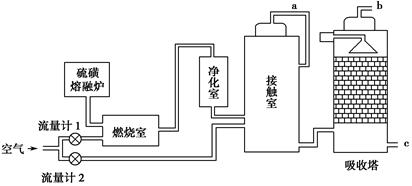
�����йع�ҵ����������ȷ����
| A����ҵ��ͨ��ʹ�õ�ⷨ�Ʊ������ơ�þ������ |
| B���ϳɰ���ҵ�У���NH3��ʱҺ�����������ڼӿ췴Ӧ���� |
| C�����Ṥҵ�У����ó�ѹ������ԭ���Ǵ������´���������� |
| D����⾫��ͭʱ������ͭ���Դ�ĸ������� |
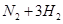


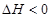 ��
��

