��Ŀ����
CaCO3��һ�ֻ���ԭ�ϣ�����ͨ����Ӧ����һϵ�����ʣ�����ͼ��ʾ��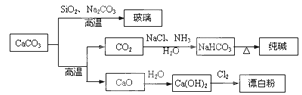
����˵����ȷ����
| A��Cl2��SO2������Ư����ɫ���ʣ���Ư��ԭ����ͬ |
B����SiO2��Na2CO3 Na2SiO3��CO2����֪��H2CO3������ǿ��H2SiO3 Na2SiO3��CO2����֪��H2CO3������ǿ��H2SiO3 |
| C����ҵ�ϣ�������ʳ��ˮ��ͨ��NH3����ͨ��CO2��NaHCO3 |
| D����ȡ�����������Ư�����漰�ķ�Ӧ���Ƿ�������ԭ��Ӧ |
C
�������������A�� Cl2��������Ư�ף�SO2Ϊ����Ư�ף�����B��������ˮ�����������ﲻ���γ���Ӧ�ᣬ�����ж϶�Ӧ�������ǿ�����÷�Ӧԭ���Dz��ӷ��������ƻӷ������ʣ�����C����ͨ�백������ͨ�������̼����̼�������ܽ�Ƚϵͣ��ʳ�����������ȷ��D����Ư����������ԭ��Ӧ������
���㣺���黯ѧ��Ӧԭ���й����⡣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���й��ڻ��������������У�����Ŀǰ��ҵ����ʵ�ʵ���
| A��ʯ��ҵ�У����ø���ķ�����ʯ�ͷֳɲ�ͬ�е㷶Χ�IJ�Ʒ |
| B�����Ṥҵ�У�Ϊ�˼ӿ찱���������ʣ�ͨ��ʹ������ý������ |
| C�����ҵ�У����ͨ�������Ͷ�����̼��ѭ�����ã������ԭ�ϵ������� |
| D���ϳɰ���ҵ�У����ø�ѹ����������ϳɰ���Ӧ��ƽ�ⳣ��������ԭ��ת���� |
�ִ���ҵ���ð��������̼�ڸ�ѹ�·�Ӧ�������ɰ�������泥�����ˮ�������ء���ӦʽΪ2NH3��CO2 NH2COONH4��NH2COONH4
NH2COONH4��NH2COONH4 CO(NH2)2��H2O
CO(NH2)2��H2O
������ѧ֪ʶ�ƶ���̵����õĻ�ѧ��Ӧ��(����)
| A��NO2��H2O�ķ�Ӧ����HNO3��NO | B��NO��O2��Ӧ����NO2 |
| C��NH3������������NO | D��N2 ������ ������ |
���й��ڻ�������ԭ���������У�������Ŀǰ��ҵ����ʵ�ʵ���
| A���ȼҵ�У����������ڵ��۵����������� |
| B����������������ڹ��������������Ȼ��⣬��ˮ���յõ����� |
| C�����������ڽӴ��ұ��������������������������������ڱ�ˮ�����Ƴ�Ũ���� |
| D���ϳɰ���ҵ�У����ð���Һ���������N2��H2ѭ��ʹ�ã�����������˰��IJ��� |
����������ԭ�ϣ��ýӴ�����������Ĺ����У��Է�����Դ�Ĵ�������ȷ���� (����)��
| A�������ᷨβ���������Ƚ�β���ð�ˮ���գ�Ȼ������Ũ���ᴦ�� |
| B����β����������ȣ���ת��������Ͷ�ʽ��٣������з�����Խϸ� |
| C�����Դ��������ų��������л���������ɫ�����Լ����ؽ�������� |
| D������¯�Կ������á����ȡ���¯���������Ȳ������������������ |
���й��ڻ�������ԭ���ļ��������У�������Ŀǰ��ҵ����ʵ���������(����)
| A��ʯ�������Ļ���ʯ��ҵ�в��ø���ķ�����ʯ�ͷֳɲ�ͬ�е㷶Χ�IJ��� |
| B����������������ڹ��������������Ȼ��⣬����ˮ������������ |
| C�����������ڽӴ��ұ��������������������������ڱ�ˮ�����Ƴ�Ũ���� |
| D���ϳɰ���ҵ�У����ڰ���Һ����N2��H2ѭ��ʹ�ã�����������˵���IJ��ʺܸ� |



