��Ŀ����
11��Ϊ�ⶨһ�ָ����������͵Ĵ��Է�ĩ���ϵ���ɣ���ȡ12.52g��Ʒ������ȫ�����ڹ���ϡ��������100.00mL��Һ��ȡ��һ�룬�������K2SO4��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӣ���ɺ��4.66g���壮�����µ�50.00mL��Һ�м�������KSCN��Һ���Ժ�ɫ������������NaOH��Һ�������ɺ��ɫ���������������ˡ�ϴ�ӡ����պ��3.20g���壮����1�����Է�ĩ��������Ԫ�ص�����������20.45%��
��2���ô��Բ�����������ϡ�������Һ�е��������б����ӡ������Ӻ������ӣ�
���� ���������֪4.66g���������ᱵ���������ټ�������еı�Ԫ�ص�������3.20g������Fe2O3����������������е���Ԫ�ص��������������������Ԫ�ص�����=12.52g-��Ԫ�ص�������2-��Ԫ�ص�������2��������Ԫ�ص���������12.52g����ѧʽʱ���ݸ�Ԫ�ص������������ԭ�����������ԭ�Ӹ���������
��� �⣺��1���������������������Һ�õ���ɫ������������ֻ�����ᱵ���ܣ�����4.66g���������ᱵ�������������ᱵ�б�Ԫ�ص�������4.66g��$\frac{137}{233}$��100%=2.74g������������м����������Ƶõ����ɫ���������ɫ����ֻ����������������3.20g��Fe2O3����������Fe2O3����Ԫ�ص�������3.20g��$\frac{112}{160}$��100%=2.24g��������Ԫ��������12.52g-2.74g��2-2.24g��2=2.56g��������Ԫ����������Ϊ$\frac{2.56}{12.52}$��100%=20.45%��
�ʴ�Ϊ��20.45%��
��2���ɱ�Ԫ������Ϊ��2.74g����Ԫ������Ϊ��2.24g����Ԫ������Ϊ��2.65g�����Ա���������ԭ�Ӹ�����Ϊ��$\frac{2.74}{137}$��$\frac{2.24}{56}$��$\frac{2.56}{16}$=1��2��4 ���Ի�ѧʽΪ��BaFe204��BaO•Fe203�����ݻ��ϴ�����Ϊ�㣬��Ϊ+3�ۣ�������������ϡ�������Һ�е��������б����ӡ������Ӻ������ӣ��ʴ�Ϊ�������ӡ������Ӻ������ӣ�
���� ���⿼�黯ѧʽ�ļ��㷽���������Ĺؼ���Ҫ֪��4.66g���������ᱵ��������3.20g����������������������

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�| A�� | 0.5��Cl2 | B�� | 1��Clԭ�� | ||
| C�� | 3.01��1023��Clԭ�� | D�� | 6.02��1023��Clԭ�� |
| A�� | �е���ת����������ԭ��Ӧ�ı��ʣ���Ԫ�ػ��ϼ۵ı仯��������ԭ��Ӧ����۱��� | |
| B�� | һ�����ʱ���������Ȼ����һ�����ʱ���ԭ | |
| C�� | �����������ʵõ���ƫ����ӣ�����ԭ������ʧȥ��ƫ����� | |
| D�� | �������ڷ�Ӧ�б���������ԭ���ڷ�Ӧ�б���ԭ |

| A�� | a��d��Ϊͬ�������� | B�� | b��c��Ϊͬϵ�� | ||
| C�� | a��d���ܷ����ӳɷ�Ӧ | D�� | ֻ��b��c�ܷ���ȡ����Ӧ |
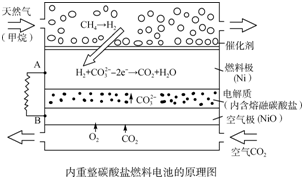
| A�� | �Դ˵��Ϊ��Դ��⾫��ͭ������0.1 mol e-ת��ʱ����3.2 gͭ�ܽ� | |
| B�� | ���Լ���Ϊȼ����ʱ��������Ӧʽ��CH4+5O2--8e-�TCO32-+2H2O | |
| C�� | �õ��ʹ�ù������貹��Li2CO3��K2CO3 | |
| D�� | �����������ĵ缫��ӦʽΪO2+4e-+2CO2�T2CO${\;}_{3}^{2-}$ |
| A�� | c��OH-����c��H+����c��B+����c��A-�� | B�� | c��B+����c��A-����c��H+����c��OH-�� | ||
| C�� | c��B+����c��A-����c��OH-����c��H+�� | D�� | c��A-����c��B+����c��H+����c��OH-�� |
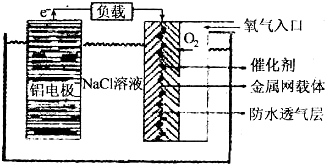
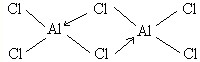 ��
�� ��
�� ��
�� ���г����µ�0.1mol/LС�մ���Һ���ش��������⣺
���г����µ�0.1mol/LС�մ���Һ���ش��������⣺