��Ŀ����
����Ŀ�����磺ͨ���ǻ���̼̼˫������ʱ���ȶ����������б仯��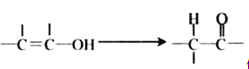 ��������ͼ��ʾ��ת����ϵ���ش�����:
��������ͼ��ʾ��ת����ϵ���ش�����:
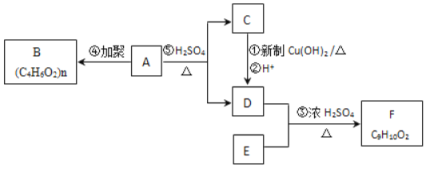
(1)A�Ļ�ѧʽ��____________________��
(2)B�Ľṹ��ʽ��____________________��
(3)�ٵĻ�ѧ����ʽ��____________________________________________________________��
(4)F�Ƿ����廯�����ұ�����ֻ��һ��������д��F�Ľṹ��ʽ____________________________________________________________________��
(5)G��F��ͬ���칹�壬�й�G�����������ܷ���ˮ�⣻�ڱ�����������ȡ�������۱�����һ�������2�֣��ݴ��Ʋ�G�Ľṹ��ʽ������(д������һ��)___________________��
���𰸡�C4H6O2 ![]() CH3CHO+2Cu(OH)2+NaOH
CH3CHO+2Cu(OH)2+NaOH![]() CH3COONa+Cu2O��+3H2O
CH3COONa+Cu2O��+3H2O 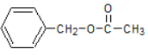
 ��
��
��������
A�����Ӿ۷�Ӧ����B��˵��A�ķ���ʽΪC4H6O2��A�������������·���ˮ�ⷴӦ��˵��A�к���������A�IJ����Ͷ�Ϊ![]() =2����֪�����к���һ��������һ��̼̼˫����C�������Ƶ�������ͭ����Һ��Ӧ��˵��C�к���ȩ������D������ͬ��̼ԭ��������C��D�ж�����2��̼ԭ�ӣ���ôCΪCH3CHO��DΪCH3COOH����ô��֪AΪCH3COOCH=CH2����BΪ
=2����֪�����к���һ��������һ��̼̼˫����C�������Ƶ�������ͭ����Һ��Ӧ��˵��C�к���ȩ������D������ͬ��̼ԭ��������C��D�ж�����2��̼ԭ�ӣ���ôCΪCH3CHO��DΪCH3COOH����ô��֪AΪCH3COOCH=CH2����BΪ![]() ��D��E����������Ӧ����F������F�ķ���ʽ��֪E�÷���ʽΪC7H8O��E�IJ����Ͷ�Ϊ
��D��E����������Ӧ����F������F�ķ���ʽ��֪E�÷���ʽΪC7H8O��E�IJ����Ͷ�Ϊ![]() 4��F�Ƿ����廯�����ұ�������һ�����������EΪ
4��F�Ƿ����廯�����ұ�������һ�����������EΪ![]() ����ôFΪ
����ôFΪ![]() ���ݴ�����
���ݴ�����
��1���ɷ�����֪�� AΪCH3COOCH=CH2 ����ѧʽ��ΪC4H6O2��
��2���ɷ���֪��B�Ľṹ��ʽ��![]() ��
��
��3����Ӧ��ΪCH3CHO�����Ƶ�������ͭ�ķ�Ӧ������ʽΪ��CH3CHO+2Cu(OH)2+NaOH![]() CH3COONa+Cu2O��+3H2O��
CH3COONa+Cu2O��+3H2O��
��4��F�Ƿ����廯�����ұ�����ֻ��һ��������д��F�Ľṹ��ʽ![]() ��
��
��5��F�Ľṹ��ʽ![]() ��G��F��ͬ���칹�壬 G�����������ܷ���ˮ�⣻�ڱ�����������ȡ�������۱�����һ�������2�֣�˵�������������ֲ�ͬ��������ԭ�ӣ�������������
��G��F��ͬ���칹�壬 G�����������ܷ���ˮ�⣻�ڱ�����������ȡ�������۱�����һ�������2�֣�˵�������������ֲ�ͬ��������ԭ�ӣ�������������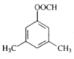 ��
�� ��
��

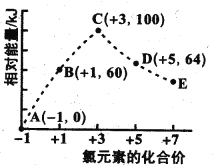
����Ŀ����.������ᣬ��Դ�ķ�չ�ѳ�Ϊȫ���繲ͬ��ע�Ļ��⣬���顢�����ѵ�ȼ���Ƚϴ�����ȼ�ϡ���ͼ1��ʾ���顢������ȼ�չ����е������仯��
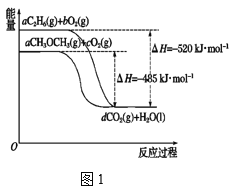
��ش��������⣺
��1��a��______��
��2�������ȼ����Ϊ______��
��3�������ʵ�����C2H6(l)��C2H6(g)��ȫȼ�������ȶ���������ʱ�ų�������______(����������������)��
��4��������ͼд����������ȫȼ��ʱ���Ȼ�ѧ����ʽ��________��
��5���ӻ����Ƕȷ������ų���ͬ������ʱѡ��_______(����������������������)��Ϊȼ�ϲ����Ķ�����̼���١�
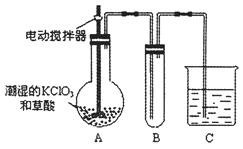
��.������ͼ2װ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL0.55mol/L NaOH ��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
��1����С�ձ��������ĭ���ϵ�������_______��
��2������NaOH��Һ����ȷ������_______��������ѡ������
A��һ��Ѹ�ٵ��� B���������������� C���ز�������������
��3��ʹ������ NaOH ��Һ��Ͼ��ȵ���ȷ������_______��
��4��ʵ���������±���
�¶�ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2��t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ��_______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 29.8 | ||
�ڽ�����Ϊ0.55mol/LNaOH��Һ��0.25mol/L ������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c��4.18J/��g���棩�����к�����H��______��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ/mol ��ƫ�����ƫ���ԭ�������________��������������