��Ŀ����
����Ŀ����ѧ�ҷ��ֶ�ұ�����е�⾫���ᴿ�ɽ��ߴ����Ʊ��ɱ�����ص��װ����ͼ��ʾ����Cu-Si�Ͻ�����Դ����950����������Һ���ν��е�⾫�����й�˵����ȷ����
A.�ڸ�Һ��������Cu������Si��������Si4+������Cu2+����ԭ
B.Һ̬Cu-Si�Ͻ��������������������
C.����ǿ�ȵĴ�С����Ӱ����ᴿ����
D.����Һ���ε������������ⷴӦ�Ӵ��������߹����Ч��
���𰸡�D
��������
��ͼ��֪��װ��Ϊ���أ�Si4+��Һ̬���缫�õ���ת��ΪSi������Һ̬���缫Ϊ���������ӵ�Դ��������Cu-Si�Ͻ����ڵ缫Ϊ���������Դ������ӣ�����Һ�����ڵ����г䵱����ʣ����Թ����������ƶ����������ⷴӦ�������߹����Ч�ʣ��ݴ˷������
A����ͼ��֪�����ص�������Siʧ����ת��ΪSi4+��������ӦΪSi4+�õ���ת��ΪSi������Si������Cu����������A����
B��ͼ�У����缫��Si4+�õ��ӻ�ԭΪSi���ʸõ缫Ϊ���������Դ������������B����
C������ǿ�Ȳ�ͬ���ᵼ��ת�Ƶ��ӵ�����ͬ����Ӱ����ᴿ���ʣ���C����
D������Һ�����ڵ����г䵱����ʣ����Թ������ƶ��������ƶ����������ⷴӦ�������߹����Ч�ʣ���D��ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����̼����������ࡣ�ش��������⣺
(1)һ���¶��£����ܱ������н������ʵ�����CO(g)��H2O(g)��ϣ������ʵ��Ĵ������з�ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)����֪���¶��£��÷�Ӧ��ƽ�ⳣ��K=16����ƽ��ʱ��ϵ��H2�����ʵ�������Ϊ_________%��
CO2(g)+H2(g)����֪���¶��£��÷�Ӧ��ƽ�ⳣ��K=16����ƽ��ʱ��ϵ��H2�����ʵ�������Ϊ_________%��
(2)�ڴ���Ru���£�CO2��H2��Ӧ������CH4����Ӧ����ʽΪCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)����֪H2������������¶ȵ����߶����ӡ����¶ȴ�300������400�棬���´ﵽƽ�⣬��v��______(��������������С����������������ͬ)��v��_______��ƽ�ⳣ��K________��ת������________��������ͬ�¶�ʱ��������Ӧ�ڲ�ͬ��ʼŨ���·ֱ�ﵽƽ�⣬�����ʵ�ƽ��Ũ�����±���
CH4(g)+2H2O(g)����֪H2������������¶ȵ����߶����ӡ����¶ȴ�300������400�棬���´ﵽƽ�⣬��v��______(��������������С����������������ͬ)��v��_______��ƽ�ⳣ��K________��ת������________��������ͬ�¶�ʱ��������Ӧ�ڲ�ͬ��ʼŨ���·ֱ�ﵽƽ�⣬�����ʵ�ƽ��Ũ�����±���
c(CO2)/mol/L | c(H2)/mol/L | c(CH4)/mol/L | c(H2O)/mol/L | |
ƽ��I | a | b | c | d |
ƽ��II | m | n | x | y |
��a��b��c��d��m��n��x��y֮��Ĺ�ϵʽΪ_____________��
(3)��֪������ʵĵ���ƽ�ⳣ�����±���
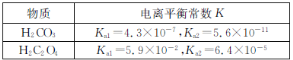
��0.1 mol��L-1��Na2CO3��Һ��pH_______(������������С��������������)0.1 mol��L-1��Na2C2O4��Һ��pH��
��������Ũ�ȵIJ�����Һ��̼����Һ�������ϣ���Һ������Ũ�ȴ�С��˳����ȷ����_________(����ĸ)��
a.c(H+)>c(HC2O4-)>c(HCO3-)>c(CO32-) b.c(HCO3-)>c(HC2O4-)>c(C2O42-)>c(CO32-)
c.c(H+)>c(HC2O4-)>c(CO32-)>c(C2O42-) d.c(H2CO3)>c(HCO3-)>c(HC2O4-)>c(CO32-)