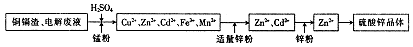��Ŀ����
1������������һ����ɫҺ�壬�ܶȱ�ˮ������ˮ���������Ҵ����۵�5.5�棬�е�267�档1�����ӣ������뱽�����ƣ��۵�96�棬�е�278�棬����ˮ���������Ҵ����Ҵ��ķе�Ϊ78.5�档1�������������������ϣ�Ҳ�ɺϳ��������ϡ�ʵ�����Ʊ�1�����������Ĺ������£�
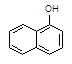 ��C2H5OH
��C2H5OH
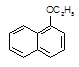 ��H2O
��H2O
1������ 1����������
��1����72g1����������100mL��ˮ�Ҵ��У�����5mLŨ�����ϡ������Һ������ͼ��ʾ�������м��ȳ�ַ�Ӧ��ʵ����ʹ�ù����Ҵ���ԭ���� ����ƿ�����ӳ�ֱ�����ܵ���Ҫ������ ��

��2����Ӧ����������ƿ�е�Һ�嵹����ˮ�У��������õ��л��㡣Ϊ�ᴿ�����������IJ�������������ˮϴ����Һ������10%��NaOH��Һ��ϴ����Һ��������ˮ�Ȼ��Ƹ��ﲢ���ˡ���ȷ��˳���� ������ţ���
A���ۢڢܢ� B���٢ڢۢ� C���ڢ٢ۢ�
��3��ʵ����1�����������IJ����뷴Ӧʱ�䡢�¶ȵı仯��ͼ��ʾ��ʱ���ӳ����¶����ߣ�1�����������IJ����½���ԭ������� �� ��

��4��ijͬѧ�Ʋ⾭�ᴿ�IJ�Ʒ���ܻ�����1�����ӡ��Ҵ��������ˮ�����ʣ���������·������м��飬���ڴ������ɱ������ݡ�
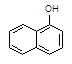 ��C2H5OH
��C2H5OH
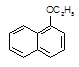 ��H2O
��H2O1������ 1����������
��1����72g1����������100mL��ˮ�Ҵ��У�����5mLŨ�����ϡ������Һ������ͼ��ʾ�������м��ȳ�ַ�Ӧ��ʵ����ʹ�ù����Ҵ���ԭ���� ����ƿ�����ӳ�ֱ�����ܵ���Ҫ������ ��

��2����Ӧ����������ƿ�е�Һ�嵹����ˮ�У��������õ��л��㡣Ϊ�ᴿ�����������IJ�������������ˮϴ����Һ������10%��NaOH��Һ��ϴ����Һ��������ˮ�Ȼ��Ƹ��ﲢ���ˡ���ȷ��˳���� ������ţ���
A���ۢڢܢ� B���٢ڢۢ� C���ڢ٢ۢ�
��3��ʵ����1�����������IJ����뷴Ӧʱ�䡢�¶ȵı仯��ͼ��ʾ��ʱ���ӳ����¶����ߣ�1�����������IJ����½���ԭ������� �� ��

��4��ijͬѧ�Ʋ⾭�ᴿ�IJ�Ʒ���ܻ�����1�����ӡ��Ҵ��������ˮ�����ʣ���������·������м��飬���ڴ������ɱ������ݡ�
| ʵ��Ŀ�� | ʵ����� | Ԥ������ͽ��� |
| ���ý����Ƽ���1�����������Ƿ� | ȡ�������ᴿ�IJ�Ʒ���Թ�A�У���������� | �� �����Ʒ������ �� �����Ʒ������ |
| �ڼ��龭�ᴿ�IJ�Ʒ�Ƿ���1������ | | �� ������1�����ӣ� �� ����1�����ӡ� |
��16�֣�
��1�����1�����ӵ�ת���ʣ������ܼ�����2�֣� �������������ԭ���Ҵ��������ʣ�2�֣�
��2��A ��2�֣�
��3��1�����ӱ�������2�֣� �¶ȸ��Ҵ������ӷ������¶ȸ߷�������Ӧ����2�֣�
��4�����������ݲ��������Ʒ������1�֣� �������ݲ��������Ʒ������1�֣�
��ȡ�������ᴿ�IJ�Ʒ���Թ�B�У�����1��2��FeCl3��Һ��2�֣�
������ɫ������1�����ӣ�1�֣� ��������ɫ����1�����ӣ�1�֣�
���ߣ�ȡ�������ᴿ�IJ�Ʒ���Թ�B�У��ȼ������Ҵ������ܽ⣬�ٵ���1��2��Ũ��ˮ��2�֣� ��������ɫ����������1�����ӣ�1�֣� ����������ɫ��������1�����ӣ�1�֣�
��1�����1�����ӵ�ת���ʣ������ܼ�����2�֣� �������������ԭ���Ҵ��������ʣ�2�֣�
��2��A ��2�֣�
��3��1�����ӱ�������2�֣� �¶ȸ��Ҵ������ӷ������¶ȸ߷�������Ӧ����2�֣�
��4�����������ݲ��������Ʒ������1�֣� �������ݲ��������Ʒ������1�֣�
��ȡ�������ᴿ�IJ�Ʒ���Թ�B�У�����1��2��FeCl3��Һ��2�֣�
������ɫ������1�����ӣ�1�֣� ��������ɫ����1�����ӣ�1�֣�
���ߣ�ȡ�������ᴿ�IJ�Ʒ���Թ�B�У��ȼ������Ҵ������ܽ⣬�ٵ���1��2��Ũ��ˮ��2�֣� ��������ɫ����������1�����ӣ�1�֣� ����������ɫ��������1�����ӣ�1�֣�
�����������1�����ȣ�����1�����ӵ��۷е��֪�������ǹ��壬�������������Ҵ������ʣ��γ�1�����ӵ��Ҵ���Һ�ܼӿ췴Ӧ���ʣ����ʹ�ù����Ҵ���ԭ�������ܼ�����Σ��Ҵ�������������1������ת���������ת���ʣ���Ȼ1�����ӡ�1�����������ķе㳬��200�棬�����Ҵ��ķе����100�棬��Ӧ�������Ҵ����ױ�������ݳ�������1�����ӵ�ת���ʽ��ͣ���ƿ�����ӳ�ֱ�����ܵ�Ŀ��������������ʹ�Ҵ�������Ϊ�Ҵ�Һ�壬���ԭ���Ҵ��������ʣ���2����ƿ��Һ�庬��1������������1�����ӡ��Ҵ��������ˮ������ˮ�еõ����л��㺬��1����������1�����ӡ��Ҵ������ڷ���������ԣ���NaOH��Һ�����кͷ�Ӧ����������ˮ�����Σ���1�����������Ҵ����Ƿǵ���ʣ�������NaOH��Һ��Ӧ������ȼ��������NaOH��Һ��ȥ1�����ӣ���ˮϴ����Һ���õ����л��㺬��1�����������Ҵ�������ˮ��Ȼ������ˮ�Ȼ��Ƴ�ȥˮ�֣�����������Һ���зе����ϴ��1�������������Ҵ��������������1�������������Ҵ������Եõ�������1��������������A��ȷ����3�����ڷ����������ױ������е������������Ҵ��ķе�ϵͣ���˷�Ӧʱ���ӳ��������¶ȣ�����1�����ӱ��������Ҵ������ӷ������Ҵ������ʷ�������Ӧ���������˷�Ӧ��Ũ�ȣ�����1�����������IJ����½�����4����1�������������������ǻ���������Na��Ӧ��1�����ӡ��Ҵ��������ˮ������Na�����û���Ӧ��������������ȡ�������ᴿ�IJ�Ʒ���Թ�A�У���������ƣ������ݲ��������Ʒ�����������ݲ��������Ʒ��������1�����Ӻ����ǻ������ǻ��뱽��ֱ��������˵�������ڷ��࣬�����뱽�����ƣ����ڱ��ӿ���Ũ��ˮ���Ȼ�����Һ���飻����һ����ȡ�������ᴿ�IJ�Ʒ���Թ�B�У�����1��2��FeCl3��Һ����Һ����ɫ������1�����ӣ���Һ������ɫ����1�����ӣ�����������ȡ�������ᴿ�IJ�Ʒ���Թ�B�У��ȼ������Ҵ������ܽ⣬�ٵ���1��2��Ũ��ˮ��������ɫ����������1�����ӣ���������ɫ��������1�����ӡ�

��ϰ��ϵ�д�
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ
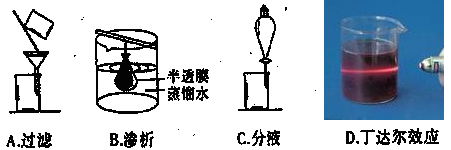
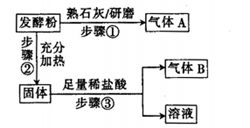
 Ԥ���������
Ԥ���������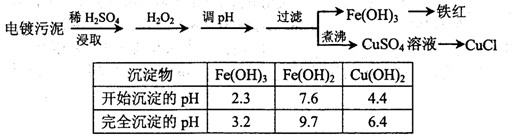
 Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��