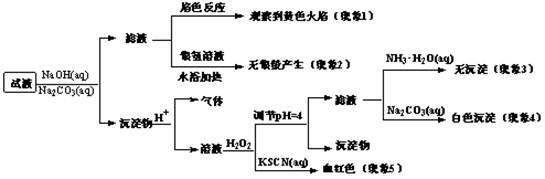
��Ŀ����
���ͷ���һ����������Ʒ����ʳƷ�Ļ�ѧ���ɼ�����С�մ���(̼�����)�������е�����������ɡ�ij�о���ѧϰС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
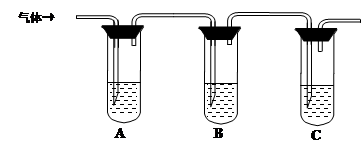
��1������1����С�մ�ͳ�����ɣ�
����2����С�մ��������ɣ�
����3����________________��ɡ�
�����������̡�
Ϊ̽��ijƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣�ijͬѧ�������ʵ�飬�õ���������
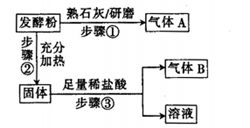
��2���÷��ͷ۵ijɷ�Ϊ________ (�ѧʽ����
��3����һƷ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2������������ʵ����֤��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��4����һƷ�Ƶķ��ͷ۵Ļ�ѧ���ΪС�մ��̼����泥�Ϊ̽���仯ѧʽ[��ѧʽ�ɱ�ʾΪnNaHCO3��m NH4HCO3]����ȡ4.05 g�ò�Ʒ���ձ����ܽ������________���100 mL��Һ������Һ��ȡ25.00mL��Һ���μ��������ᣬ���ɵ�����ͨ���������ʯ��ˮ�У����ɵİ�ɫ�������������Ϊ1.25 g����÷��ͷ۵Ļ�ѧʽΪ ��
����Է���������NaHCO3��84 NH4HCO3��79 CaCO3��100��
��������衿
��1������1����С�մ�ͳ�����ɣ�
����2����С�մ��������ɣ�
����3����________________��ɡ�
�����������̡�
Ϊ̽��ijƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣�ijͬѧ�������ʵ�飬�õ���������

��2���÷��ͷ۵ijɷ�Ϊ________ (�ѧʽ����
��3����һƷ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2������������ʵ����֤��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� |  Ԥ��������� Ԥ��������� |
| 1��ȡ������Ʒ����ϡ�������Һ�ֳ����� | |
| 2�� _______________________________________ | |
| 3�� ________________________________________ | |
��4����һƷ�Ƶķ��ͷ۵Ļ�ѧ���ΪС�մ��̼����泥�Ϊ̽���仯ѧʽ[��ѧʽ�ɱ�ʾΪnNaHCO3��m NH4HCO3]����ȡ4.05 g�ò�Ʒ���ձ����ܽ������________���100 mL��Һ������Һ��ȡ25.00mL��Һ���μ��������ᣬ���ɵ�����ͨ���������ʯ��ˮ�У����ɵİ�ɫ�������������Ϊ1.25 g����÷��ͷ۵Ļ�ѧʽΪ ��
����Է���������NaHCO3��84 NH4HCO3��79 CaCO3��100��
��17�֣�
��1�����ۺ����� ��2�֣�
��2��NH4HCO3��NaHCO3 ��2�֣�
��3����8�֣�
��4��100mL����ƿ�� 2�֣� 2NaHCO3��3NH4HCO3 (3��)
��1�����ۺ����� ��2�֣�
��2��NH4HCO3��NaHCO3 ��2�֣�
��3����8�֣�
| ʵ�鲽�� |  Ԥ��������� Ԥ��������� |
| 1��ȡ������Ʒ����ϡ�������Һ�ֳ����� | |
| 2���ù���������˿��1�֣�պȡ����һ����Һ�ھƾ����������գ��۲������ɫ��1�֣�____ | ����ʻ�ɫ��1�֣����÷��ͷ��к���NaHCO3��1�֣� |
| 3�� ������һ����Һ�еμ�������1�֣�BaCl2��Һ��1�֣��� ��������1��2�ξ��ɵ�1�֣� �Լ�BaCl2�÷֣�Ba��NO3��2��Ba��0H��2���÷֣���д����HCl�ữ���۷֣� | �а�ɫ�������ɣ�1�֣�����ϲ���2�Ľ��ۼ������1�֣����� |
��4��100mL����ƿ�� 2�֣� 2NaHCO3��3NH4HCO3 (3��)
�����������1�����������֪����ͬƷ�Ƶķ��ͷۿ���С�մ�ͳ�����ɣ�Ҳ������С�մ��������ɣ��������ɳ��ۺ�������ɣ�������֪�ļ���1������2�ƶϼ���3Ϊ���ۺ���������2������NaHCO3��NH4HCO3��Al2(SO4)2?12H2O����Ҫ���ʿ�֪��NH4HCO3����Σ�����ʯ�һ����ĥ���Էų���������������������ʯ����ĥ�����ܷų����壬��AΪNH3�����ͷۼ�һ�����г��ۣ�NH4HCO3�����ּ��Ⱥ���ȫ��Ϊ�����ݳ���NaHCO3���������ȱ�ΪNa2CO3�����CO2��H2O��Na2CO3������������Ӧ���ɶ�����̼���塢NaCl��H2O����Al2(SO4)2?12H2O����ˮ�����Al(OH)3�������϶�������������ɴ��ƶ�BΪCO2���÷��ͷ�һ������С�մ����Լ�Ʒ�Ƶķ��ͷ۵���Ҫ�ɷ���NaHCO3��NH4HCO3����3������ʵ�鷽���в���2�Ľ������ƿ�֪������2���ýྻ�IJ�˿պȡA�е���Һ���پƾ������������գ��������ɫ�ʻ�ɫ��֤����Na+�����ͷ�����NaHCO3�����ڼ���2�Ƿ��ͷ���С�մ��������ɣ�����3�Ľ�����֤�����ͷ���Al2(SO4)2?12H2O�����������ʼ��ṩ�Լ��������ƶϣ�����3�����ʵ�鷽��֤�����ͷ��к���Al3+�����Ӧ��B�Թ�����εμ�0.1mol/LNaOH��Һ���۲쵽��ɫ�������Ȳ�����ɫ����������ܽ⣬֤����Al3+�����ͷ�������������4��������Һ��Ҫ���������������ܽ⡢ת�ơ�ϴ�ӡ����ݵȲ��裬������100 mL��Һ��Ҫ���ܽ⡢��ȴ�����Һ����100mL����ƿ�У�25.00mL������ȫ��Ӧ������CO2+Ca(OH)2=CaCO3��+H2O�����ɫ����Ϊ̼��ƣ�����n=m/M����n(CaCO3)=1.25g��100g/mol=0.0125mol������100mL������Һ�������25.00mL������Һ֮��Ϊ100/25.00����100mL������Һ���������ᷴӦ�ų��Ķ�����̼Ϊ0.0125mol��100/25.00=0.05mol���跢�ͷ���NaHCO3��NH4HCO3�ֱ�Ϊxmol��ymol������m=n��M����84x+79y=4.05����������������NaHCO3+HCl=NaCl+CO2��+H2O��NH4HCO3+HCl=NH4Cl+CO2��+H2O����xmolС�մ���ȫ��Ӧ�ų�xmol������̼���壬ymol̼�������ȫ��Ӧ�ų�ymol������̼����x+y=0.05����������������⣬��x=0.02��y=0.03��˵���÷��ͷ���С�մ��̼����淋����ʵ���֮��Ϊ0.2��0.3=2��3�����Ը÷��ͷ۵���ɿ��Ա�ʾΪ2NaHCO3��3NH4HCO3��

��ϰ��ϵ�д�
�����Ŀ

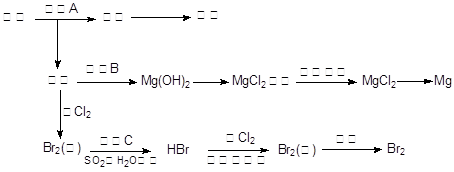
 I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�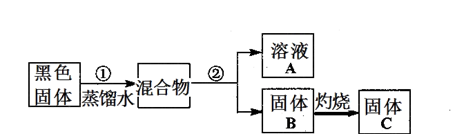
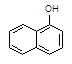 ��C2H5OH
��C2H5OH
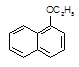 ��H2O
��H2O