��Ŀ����
ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�
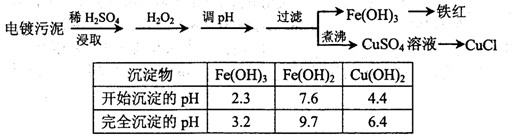
��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ ���ѧʽ����ʵ���ҽ��й��˲������õ��IJ��������� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��CuCl��Ʒ��CuCl��������������96��50%Ϊ���Һϸ������ȡ���Ʊ���CuCl��Ʒ0��2500g����һ������0��5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1��Ce��SO4��2��Һ�ζ��������յ�ʱ����Ce��SO4��2��Һ24��60mL���йصĻ�ѧ��ӦΪ��
Fe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3����ͨ������˵����CuCl��Ʒ ������ϡ������ϡ������ұ���
��4��25��ʱ��KSP [Fe��OH��3]= 4.0��10-38��Fe3+����ˮ�ⷴӦFe3����3H2O Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��

��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ ���ѧʽ����ʵ���ҽ��й��˲������õ��IJ��������� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��CuCl��Ʒ��CuCl��������������96��50%Ϊ���Һϸ������ȡ���Ʊ���CuCl��Ʒ0��2500g����һ������0��5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1��Ce��SO4��2��Һ�ζ��������յ�ʱ����Ce��SO4��2��Һ24��60mL���йصĻ�ѧ��ӦΪ��
Fe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3����ͨ������˵����CuCl��Ʒ ������ϡ������ϡ������ұ���
��4��25��ʱ��KSP [Fe��OH��3]= 4.0��10-38��Fe3+����ˮ�ⷴӦFe3����3H2O
 Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ����14�֣���1����Fe2+������Fe3�������ڵ���pHֵ��Cu2+���루2�֣���
CuO��Cu(OH)2��CuCO3�ȣ�2�֣���©�����ձ�����������2�֣�
��2��������Һ�е�H2O2������Ӱ����һ��CuCl�����ɣ�2�֣�
2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4��2�֣�
��3�����ϣ�2�֣� ��4��2.5��10��5��2�֣�
CuO��Cu(OH)2��CuCO3�ȣ�2�֣���©�����ձ�����������2�֣�
��2��������Һ�е�H2O2������Ӱ����һ��CuCl�����ɣ�2�֣�
2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4��2�֣�
��3�����ϣ�2�֣� ��4��2.5��10��5��2�֣�
�����������1����������к���ͭ�����Ƚ���������������Ҫ���ܽ�ͭ�����Ƚ��������˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+���л�ԭ�ԣ���������H2O2��Fe2+�ܱ�˫��ˮ����ΪFe3�������ڵ���pHֵ��Cu2+���룻�ڵ���pH�����м�����Լ���������ȥ��Һ�е��ᣬ�Ҳ��������µ����ʣ�����Ҫ������Լ�������CuO��Cu(OH)2��CuCO3�����ݱ������ݿ�֪������Һ��pHֵ4.4ʱ��ͭ���ӿ�ʼ���ֳ���������Һ��pHֵΪ3.2ʱ�����������ӳ�����ȫ��ͭ����δ��������������Ҫʹ���������Ӻ�ͭ���ӷ��룬��Һ��pHӦ����3.2��pH��4.4.���ɵ�����������������ͨ������ʵ�ַ��룬���˲��õ�������������̨��©�����ձ����������ȣ��������ڲ���������©�����ձ�����������
��2����Ӧ�й��������ǹ����ģ�����������������ԣ��������ȥ��Ӱ��CuCl�����ɡ�˫��ˮ�����ֽ⣬�������CuSO4��Һ��ԭ���dz�����Һ�е�H2O2������Ӱ����һ��CuCl�����ɣ���ΪCuSO4�У�2�۵�ͭ�ܰ�Na2SO3�У�4�۵��������ɣ�6�۵�����2�۵�ͭ����ԭ���ɣ�1�۵�ͭ����������CuCl���������Ը÷�Ӧ�Ļ�ѧ����ʽΪ2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4��
��3������Ʒ��CuCl������Ϊx���йصĻ�ѧ��ӦΪFe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3�������ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n(CuCl) 24.60��10-3L��0.1000 mol/L
��� n(CuCl)��24.60��10-3L��0.1000 mol/L��2.46��10-3mol
���Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol��0.2448g
������Ʒ��CuCl������������
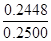 ��100%��97.91%��96.50%�����Ը���Ʒ��CuCl�������������ϱ���
��100%��97.91%��96.50%�����Ը���Ʒ��CuCl�������������ϱ�����4��KSP [Fe(OH)3]��c(Fe3+)��c3(OH-)��4.0��10-38��c(H+)��
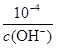 ����˷�ӦFe3����3 H2O
����˷�ӦFe3����3 H2O Fe(OH)3��3H����ƽ�ⳣ��K��
Fe(OH)3��3H����ƽ�ⳣ��K��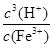 ��
��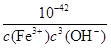 ��2.5��10-5��
��2.5��10-5��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
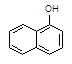 ��C2H5OH
��C2H5OH
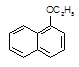 ��H2O
��H2O

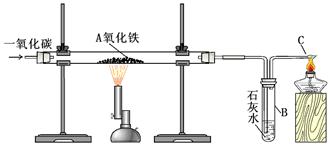
 2Fe3O4+CO2
2Fe3O4+CO2 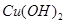 ����Һ
����Һ