题目内容
【题目】以下工业流程制备无机功能材料MnO2,粗MnO2的提纯是工业生产的重要环节,某研究性学习小组设计了将粗MnO2(含有较多MnO和MnCO3)样品转化为纯MnO2实验。
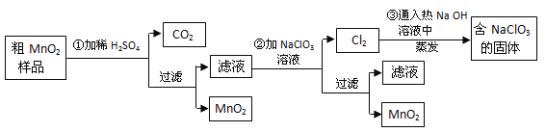
针对上述工业流程,下列选项错误的是( )
A.第①步加稀![]() 时,粗MnO2样品中的MnO、MnCO3转化为可溶性物质
时,粗MnO2样品中的MnO、MnCO3转化为可溶性物质
B.不考虑操作过程中的损失,本流程中的![]() 固体能全部循环,不需额外补充
固体能全部循环,不需额外补充
C.第②步反应的离子方程式![]()
D.实验室模拟上述工业流程中得到MnO2固体的操作必须用到的玻璃仪器有玻璃棒、烧杯、漏斗
【答案】B
【解析】
粗MnO2(含有较多的MnO2和MnCO3)样品加入硫酸,MnO2不溶于硫酸,所以加稀硫酸时样品中的MnO和MnCO3分别和硫酸反应生成可溶性的MnSO4,同时产生二氧化碳,向硫酸锰中加入氯酸钠,反应的离子方程式5Mn2++2ClO3-+4H2O=5MnO2↓+Cl2↑+8H+,将产生的氯气和热的氢氧化钠溶液反应可以得到NaCl和氯酸钠的溶液,蒸发浓缩结晶可以得到氯酸钠的固体循环利用;
A.MnO2不溶于硫酸,所以加稀硫酸时样品中的MnO和MnCO3分别和硫酸反应生成可溶性的MnSO4,故A正确;
B.Cl2通入热的NaOH溶液中除生成NaClO3,还生成NaCl,则流程中NaClO3固体不可能完全循环利用,故B错误;
C.第②步MnSO4要转化为MnO2,需失去电子,故需要加入NaClO3做氧化剂,依据得失电子守恒可以配平,所以反应的化学方程式是:5MnSO4+2NaClO3+4H2O=5MnO2+Cl2↑+Na2SO4+4H2SO4,故C正确;
D.过滤所需的仪器有烧杯、漏斗、玻璃棒,流程中得到MnO2固体操作必需的玻璃仪器有玻璃棒、烧杯、漏斗,故D正确;
故答案为B。

【题目】根据实验操作及现象所得出的解释或结论不正确的是( )
选项 | 实验操作及现象 | 解释或结论 |
A. | 向某溶液中加入足量稀盐酸,无明显现象,再加入BaCl2溶液,有白色沉淀产生 | 该溶液中一定含有SO |
B. | 向某溶液中加入浓NaOH溶液,加热,产生 能使湿润的红色石蕊试纸变蓝的气体 | 该溶液中一定含有NH |
C. | 向某钾盐中滴加盐酸,产生使澄清石灰水变浑浊的无色无味气体 | 该钾盐是K2CO3或KHCO3 |
D. | 酸性硝酸铁溶液中加入几滴碘化钾淀粉溶液,出现蓝色 | 铁离子具有较强氧化性,将I-氧化生成I2 |
A.AB.BC.CD.D
【题目】硫及其化合物有许多用途,相关物质的物理常数如下表所示:
H2S | S8 | FeS2 | SO2 | SO3 | H2SO4 | |
熔点/℃ | -85.5 | 115.2 | >600(分解) | -75.5 | 16.8 | 10.3 |
沸点/℃ | -60.3 | 444.6 | -10.0 | 45.0 | 337.0 |
回答下列问题:
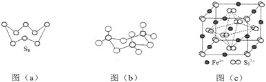
(1)基态S原子电子占据最高能级的电子云轮廓图为__________形。
(2)图(a)为S8的结构,其熔点和沸点要比二氧化硫的熔点和沸点高很多,主要原因为________。
(3)气态三氧化硫以单分子形式存在,其分子的立体构型为__________形,其中共价键的类型有__________种;固体三氧化硫中存在如图(b)所示的三聚分子,该分子中S原子的杂化轨道类型为__________。
(4)根据价层电子对互斥理论,H2S、SO2、SO3的气态分子中,中心原子价层电子对数不同于其他分子的是__________。
(5)FeS2晶体的晶胞如图(c)所示。晶胞边长为a nm、FeS2相对式量为M,阿伏加德罗常数的值为NA,其晶体密度的计算表达式为____________g/cm3;晶胞中Fe2+位于![]() 所形成的正八面体的体心,该正八面体的边长为__________nm。
所形成的正八面体的体心,该正八面体的边长为__________nm。
晶胞有两个基本要素:
(6)晶胞的一个基本要素:原子坐标参数,表示晶胞内部各原子的相对位置,下图为Ge单晶的晶胞,其中原子坐标参数A为(0,0,0);B为(![]() ,0,
,0,![]() );C为(
);C为(![]() ,
,![]() ,0)。则D原子的坐标参数为__________。
,0)。则D原子的坐标参数为__________。
【题目】Ⅰ下列单元操作中采用了热交换设计的有
A.电解食盐水制烧碱 |
B.合成氨中的催化合成 |
C.硫酸生产中的催化氧化 |
D.氨碱法中的氨盐水碳酸化 |
Ⅱ海水晒盐的卤水中还有氯化镁,以卤水为原料生产镁的一中工艺流程如下图所示。
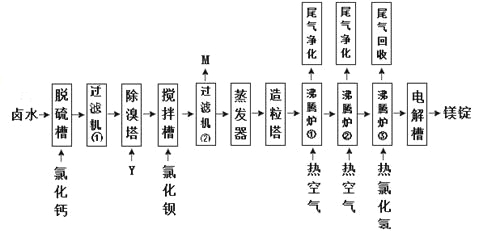
回答下列问题:
(1)脱硫槽、搅拌槽均用于脱除卤水中的(填离子符号),M的主要成分是(填化学式)。
(2)除溴塔中主要的离子方程式为。
(3)沸腾炉①和②的主要作用是。沸腾炉③通入热氯化氢的主要目的是。
(4)电解槽中阴极的电极反应方程式为。
(5)电解槽中阳极产物为,该产物可直接用于本工艺流程中的。