��Ŀ����
�����谷����ѧʽ��C3N6H6����һ�ַ�ʳƷ�����Ҫ�л�����ԭ�ϣ��㷺�������ϡ���ֽ�����ĵ���ҵ����ͼ���ҹ��Ƽ���������2004�����Ƶ�������Ϊԭ�����������谷�Ĺ��ա�������ѹ����һ�������������¼�������
��֪�������ص��۵���132.7�棬��ѹ�³���160�漴�ɷֽ⣻
�������谷���۵���354�棬����������������ˮ��
��������Ϊԭ�����������谷��ԭ���ǣ�
6CO(NH2)2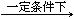 C3N6H6 + 6NH3 + 3CO2
C3N6H6 + 6NH3 + 3CO2
��ش�
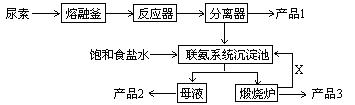
��1��������һ�ֳ��ú�������ߵĻ��ʣ��䵪Ԫ�ص������ٷ���Ϊ ����ʵ����ʹ���ۻ����������ƽ�_________ ����ҵ�Ϻϳ����صĻ�ѧ��Ӧ����ʽΪ___________________________________(��Ӧ�������Բ�д)
��2��д������Ҫ�ɷݵĻ�ѧʽ����Ʒ1 ����Ʒ2 ��X ��
��3������ϵͳ�������з����Ļ�ѧ��Ӧ����ʽΪ�� ��
��4����������������4%��������ģ�ÿ�����ؿ����������谷 �֣�����Ʒ���� �֡�

��֪�������ص��۵���132.7�棬��ѹ�³���160�漴�ɷֽ⣻
�������谷���۵���354�棬����������������ˮ��
��������Ϊԭ�����������谷��ԭ���ǣ�
6CO(NH2)2
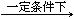 C3N6H6 + 6NH3 + 3CO2
C3N6H6 + 6NH3 + 3CO2��ش�
��1��������һ�ֳ��ú�������ߵĻ��ʣ��䵪Ԫ�ص������ٷ���Ϊ ����ʵ����ʹ���ۻ����������ƽ�_________ ����ҵ�Ϻϳ����صĻ�ѧ��Ӧ����ʽΪ___________________________________(��Ӧ�������Բ�д)
��2��д������Ҫ�ɷݵĻ�ѧʽ����Ʒ1 ����Ʒ2 ��X ��
��3������ϵͳ�������з����Ļ�ѧ��Ӧ����ʽΪ�� ��
��4����������������4%��������ģ�ÿ�����ؿ����������谷 �֣�����Ʒ���� �֡�
��1��46.7%��1�֣� ���� ��1��2NH3 + CO2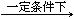 CO(NH2)2+ H2O��1�֣�
CO(NH2)2+ H2O��1�֣�
��2��C3N6H6 NH4Cl CO2��3�֣�
��3��NaCl + NH3 + CO2 + H2O �� NaHCO3�� + NH4Cl��2�֣�
��NH3 + CO2 + H2O �� NH4HCO3 NaCl + NH4HCO3 �� NaHCO3�� + NH4Cl
��4��0.336t ��1�֣� 0.848 t��1�֣�
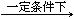 CO(NH2)2+ H2O��1�֣�
CO(NH2)2+ H2O��1�֣� ��2��C3N6H6 NH4Cl CO2��3�֣�
��3��NaCl + NH3 + CO2 + H2O �� NaHCO3�� + NH4Cl��2�֣�
��NH3 + CO2 + H2O �� NH4HCO3 NaCl + NH4HCO3 �� NaHCO3�� + NH4Cl
��4��0.336t ��1�֣� 0.848 t��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ


 �鷽���� ��
�鷽���� ��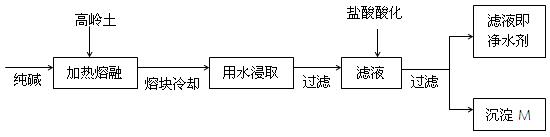
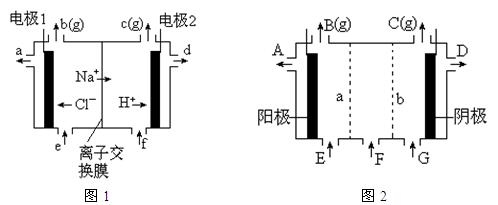
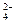 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa�� 2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺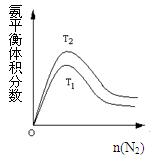
 2(g)+CO(g) ��ƽ�ⳣ��K1
2(g)+CO(g) ��ƽ�ⳣ��K1