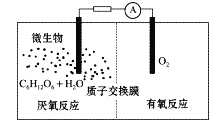
��Ŀ����
����Ŀ��С�������ȷ�ʱ��������ø��������ˮ��Ϊ��ѿ�ǡ������ǵȡ�С�������ȷ��������¶�Ϊ30�����ҡ�ȡ����С��������20����30��������������4��������´�������3֧�Թ��м����������Լ���������50�����ң�ҡ�Ⱥ�۲��Թ��е���ɫ�������(����)

A.�׳���ɫ���ҳ�ש��ɫ��������ɫ
B.�׳���ɫ���ҳ�ש��ɫ��������ɫ
C.�ס��ҽԳ���ɫ������ש��ɫ
D.�׳�dzש��ɫ���ҳ�ש��ɫ��������ɫ
���𰸡�D
��������
����С�������ȷ�ʱ��������ø��������ˮ��Ϊ��ѿ�ǡ������ǵȡ�С�������ȷ��������¶�Ϊ30�����ҡ�ȡ����С��������20��������������4��������Ļ�ԭ���DZ�30��������������4��������Ļ�ԭ����������3֧�Թ��м����������Լ���������50�����ң�ҡ�Ⱥ�۲��Թ��е���ɫ������Ǽ�����ԭ�����٣���dzש��ɫ��������ԭ���Ƕࣩ��ש��ɫ����������ˮ������ɫ��D��ȷ��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�����Ŀ����1����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H1����234.1 kJ��mol��1��
C(s)��O2(g��CO2(g) ��H2����393.5 kJ��mol��1��
��Fe��O2��Ӧ����Fe2O3���Ȼ�ѧ��Ӧ����ʽΪ_______________________________��
(2)��֪H2A��ˮ�д�������ƽ�⣺H2A��H����HA����HA��![]() H����A2����
H����A2����
�ٳ�����NaHA��Һ��pH________(�����)��ԭ����____________________________��
A������7 B��С��7 C������7 D����ȷ��
����֪������H2A�ĸ���(CaA)�ı�����Һ�д�������ƽ�⣺CaA(s)![]() Ca2��(aq)��A2��(aq) ��H>0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��______________________��
Ca2��(aq)��A2��(aq) ��H>0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��______________________��
A�������¶� B�������¶� C������NH4Cl���� D������Na2A����
(3)ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ����0.10 mol��L��1 NaOH����Һ���вⶨ�����Ũ�ȵ�ʵ�顣ȡ20.00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
ʵ���� | NaOH��Һ��Ũ��(mol��L��1) | �ζ����ʱ��NaOH��Һ��������(mL) | ������������(mL) |
1 | 0.10 | 22.65 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.93 | 20.00 |
�����������գ�
�ٵζ��ﵽ�յ�ı�־��_____________________________________________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ_____________________(������λ��Ч����)��
��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ���________(����ĸ���)��
A���ζ��յ����ʱ����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C����ƿˮϴ��δ����
D������NaOH�������Na2CO3����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ