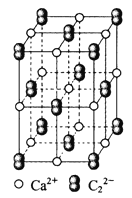
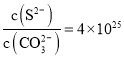��Ŀ����
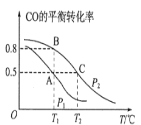
����Ŀ����CO2��һ����������H2��Ӧת��Ϊ�״�(CH3OH)�DZ��Ϊ���ĺ÷�����һ�������£�ÿת��44g CO2�ų�������Ϊ49 kJ��CO2ת��Ϊ�״�������Ũ����ʱ��ı仯������ͼ��ʾ(��֪��Ӧ����������ڴ������¾�Ϊ����)��������������ȷ����( )
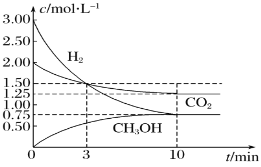
A.0��3 min�ڣ�CO2��H2�������ƽ����Ӧ������ȣ���Ϊ0.5 mol��L-1��min-1
B.�˷�Ӧ���Ȼ�ѧ����ʽΪCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����H=��49.0 kJ��mol-1
CH3OH(g)+H2O(g)����H=��49.0 kJ��mol-1
C.�������·�Ӧ��ƽ�ⳣ��K=![]()
D.�����¶ȣ��˷�Ӧ��ƽ�ⳣ������Ϊ0.8
���𰸡�B
��������
A.��3minʱ��CO2��H2��3����ʱŨ����ȣ������ʷֱ�Ϊv(CO2)=![]() =
=![]() mol/(L��min)��v(H2)=
mol/(L��min)��v(H2)=![]() =
=![]() mol/(L��min)���������ʲ�ͬ��A����
mol/(L��min)���������ʲ�ͬ��A����
B.���ȴ�ͼ��ɿ�������Ӧ��ΪH2��CO2(Ũ�Ƚ���)��������ΪCH3OH���ٴ�Ũ�ȵı仯������������ȿ�ȷ�����ǵ�ϵ��Ϊ3��1��1����������غ�ԭ������֪�������л���1molH2O��ÿת��44g(��1mol)CO2�ų�49.0kJ�������������Ȼ�ѧ����ʽΪ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H=-49.0kJ/mol��B��ȷ��
CH3OH(g)+H2O(g) ��H=-49.0kJ/mol��B��ȷ��
C.����ͼ���и������ʵ�ƽ��Ũ�ȿ�֪��Ӧ��ƽ�ⳣ��K=![]() =1.07��C����
=1.07��C����
D.�÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ�����ƽ�ⳣ������(>1.07)�����Բ�����Ϊ0.8��D����
�ʺ���ѡ����B��

����Ŀ����ȼ��(��Ȼ��ˮ�������CH4xH2O��ʾ)�Ŀ��ɺ����ã��������ڽ���������ٵ���ԴΣ������������һϵ�еĹ�ҵ��Ʒ��
(1)��ȼ����һ���������ܹ��ͷų�CH4���壬��������________(�������¡���ѹ���������¡���ѹ��)��
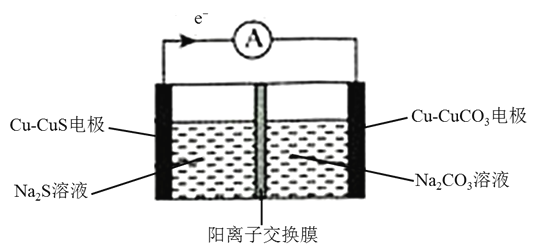
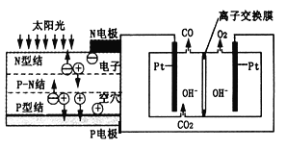
(2)����̼������ȼ�ϵ��(MCFC)ͨ������Ϊ�ڶ���ȼ�ϵ�ء���CH4ΪMCFC��ȼ��ʱ����صĹ���ԭ����ͼ��ʾ��
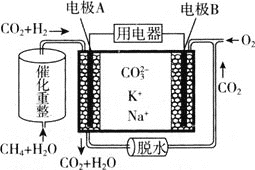
��CH4(g)��H2O(g)���ڵ���ڲ���������Ӧת��ΪH2(g)��CO2(g)����֪CH4��H2�ı�ȼ���ȷֱ�Ϊ890.3kJ/mol��285.8kJ/mol��H2O(l)=H2O(g) H=+41 kJ/mol�������������Ӧ���Ȼ�ѧ����ʽΪCH4(g)+2H2O(g)=4H2(g)+CO2(g) H=________kJ/mol��
��ͼ�е缫AΪȼ�ϵ�ص�________(��������������������)���缫B�ϵĵ缫��ӦʽΪ________��
(3)��ѹǿΪp�ĺ�ѹ�����У�CH4�ڵ绡¯����������ȡ��Ȳ����ѧ����ʽΪ2CH4=C2H2+3H2���±�Ϊ��Ӧ��ϵ�����Ϻ������
�ɷ� | ���� | ���� | ||
��CH4 | CH4 | C2H2 | H2 | |
���ʵ���(mol) | 44.8 | 19.13 | 8.96 | 39.5 |
�ټ����֪C2H2�IJ�����=________��
�ڳ�����C2H2��H2�����ʵ���֮�Ȳ�������1:3�����ܵ�ԭ����________��