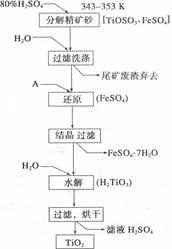
��Ŀ����
��8�֣�ij��ѧ����С���ú���Ϊԭ����ȡ������ˮ����֪�������е�Ԫ����I����ʽ���ڡ���ȡ���ԭ���ǡ���������������H2O2��I������Ϊ�ⵥ�ʡ���
��Ӧ���ٽ�������ʵ�鲽�裺
�ٰ�ʢ����Һ�ķ�Һ©����������̨����Ȧ�У��ڰ�50mL��ˮ��15mL CCl4�����Һ©���У��Ǻò���������ת©������������ʱ�����������������رջ����ѷ�Һ©���������ۼ����Һ©���������ϿڵIJ������Ƿ�©Һ����������Һ©�����������ձ������²�Һ�壻�ݴӷ�Һ©���Ͽڵ����ϲ�Һ�壻��©���ϿڵIJ�������ʹ���ϰ��۶�©�����ϵ�С�ף��߾��ã��ֲ㡣
�ʹ�ʵ�飬���������գ�
��1��д���ú�����ȡ���ʵ�����ӷ���ʽ��______________ ______________________ ��
��2����ȷ��ʵ���������˳��Ϊ�� ® ® ®��®��®��®�ݡ�
�ǵڢڲ�������������_______ _____��
�ȵڢڲ��У������������CCl4���ܼ��У�
��Ӧ���ٽ�������ʵ�鲽�裺
�ٰ�ʢ����Һ�ķ�Һ©����������̨����Ȧ�У��ڰ�50mL��ˮ��15mL CCl4�����Һ©���У��Ǻò���������ת©������������ʱ�����������������رջ����ѷ�Һ©���������ۼ����Һ©���������ϿڵIJ������Ƿ�©Һ����������Һ©�����������ձ������²�Һ�壻�ݴӷ�Һ©���Ͽڵ����ϲ�Һ�壻��©���ϿڵIJ�������ʹ���ϰ��۶�©�����ϵ�С�ף��߾��ã��ֲ㡣
�ʹ�ʵ�飬���������գ�
��1��д���ú�����ȡ���ʵ�����ӷ���ʽ��______________ ______________________ ��
��2����ȷ��ʵ���������˳��Ϊ�� ® ® ®��®��®��®�ݡ�
�ǵڢڲ�������������_______ _____��
�ȵڢڲ��У������������CCl4���ܼ��У�
| A������ | B���ƾ� | C���� | D��ˮ |

��2I�D��2H����2H2O=I2��2H2O,��H2O2�����������½�I�D������I2��
�Ʒ�Һ©��Ӧ�Ȳ�©����װҺ��Ȼ�����©�����ϣ�����˳��Ϊ�ۢڢ٣�
�Ǵӵ�ˮ����ȡ�⣬��ȡ��
��AC��BD����ˮ���ܣ����ֲ㣬��������ȡ��������

��ϰ��ϵ�д�
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
�����Ŀ



 2Na + Cl2��
2Na + Cl2�� 2Fe + 3CO2��
2Fe + 3CO2��
 4Ag + O2��
4Ag + O2�� ��д��һ�����ɣ���
��д��һ�����ɣ���