��Ŀ����
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
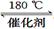
��1�����ü������ԭ���������֪��
CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H ����574 kJ/mol
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ��2NH3(g)��NO(g)��NO2(g) 2N2(g)��3H2O(g)����H < 0
2N2(g)��3H2O(g)����H < 0
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
��3������ClO2�������������ת���������£�
NO NO2
NO2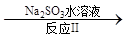 N2
N2
��֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O �� NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4������CO����ԭ��������Ҳ���Դﵽ������Ⱦ��Ŀ�ġ�
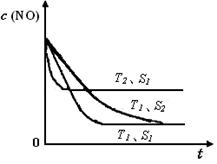
��֪����һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ2NO(g) + 2CO(g) 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
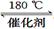
��1�����ü������ԭ���������֪��
CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H ����574 kJ/mol
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ��2NH3(g)��NO(g)��NO2(g)
 2N2(g)��3H2O(g)����H < 0
2N2(g)��3H2O(g)����H < 0Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
��3������ClO2�������������ת���������£�
NO
 NO2
NO2 N2
N2��֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O �� NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4������CO����ԭ��������Ҳ���Դﵽ������Ⱦ��Ŀ�ġ�
��֪����һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ2NO(g) + 2CO(g)
 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
��1��CH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g) ��H ����867 kJ/mol ��3�֣�
��2������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȣ�������Ҳ�Ʒ֣�
��3��2NO2 + 4 Na2SO3 = N2 + 4 Na2SO4 67.5
��4���� ��
��2������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȣ�������Ҳ�Ʒ֣�
��3��2NO2 + 4 Na2SO3 = N2 + 4 Na2SO4 67.5
��4���� ��
�����������1������֪�������Ȼ�ѧ����ʽ��ӳ���2���ã���ΪCH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g) ��H ����867 kJ/mol
��2����ߵ��������ת���ʼ�ʹ��Ӧ������У�������������ԭ�����ɲ�ȡ����NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȴ�ʩ��
��3����Ӧ���з�Ӧ��ΪNO2��Na2SO3������֮һ��N2������������ԭ��Ӧ���ۣ����ƶ���һ����ΪNa2SO4��ѧ����ʽ��2NO2 + 4 Na2SO3 = N2 + 4 Na2SO4��������11.2 L N2����״�����������ʵ���Ϊ0.5mol������NO2�����ʵ���Ϊ1mol,�Ӷ��ɼ��������ClO2������Ϊ67.5g;
��4�����ݻ�ѧƽ���еġ��ȹ���ƽ�����ɣ��¶ȸߵ��ȴ�ƽ�⣬�����������ʿ죬�ȴ�ƽ�⣬����T2>T1,S1>S2,���¶����ߣ�CO��Ũ������˵������ƽ�������ƶ��������Ƿ��ȷ�Ӧ��H<0��

��ϰ��ϵ�д�
�����Ŀ

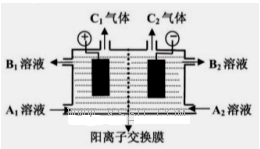



 3Cu2++2R+yH2O��
3Cu2++2R+yH2O��
 6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��