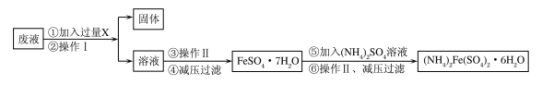
��Ŀ����
����Ŀ�����������(KBe2BO3F2)�Ǽ������ĺ��IJ���,�ҹ���ѧ���ڴ�������о��������������ǰ�С���ش��������⣺
��1����һ�����ܽ���B��N֮��ĵڶ����ڵ�Ԫ�ع���________�֡�
��2����̬K+���ӵ���ռ������ܼ��ĵ���������ͼΪ_________�Ρ�
��3��BeCl2�еĻ�ѧ���������ԵĹ����ԣ�����״̬��BeCl2��˫�۷��Ӵ��ڣ���ṹʽΪ________������Beԭ�ӵĵ����Ų�ͼΪ_________��
��4���ķ�������(NaBF4)�Ƿ�֯��ҵ�Ĵ������������ӵ�����ԭ�ӵ��ӻ��������Ϊ_________���ķ��������д���_______(�����)��
a. ��� b. ���»��� c. ���Ӽ� d. ��λ�� e. ���� f. ����
���𰸡�3 ���� ![]()
![]() sp3 c d e
sp3 c d e
��������
��1��ͬһ�����У������ң�Ԫ�صĵ�һ�����ܳʾ��״���ߣ����е�II A��͵�V A���Ԫ�صĵ�һ������Ҫ��������ڵ�����Ԫ�أ��ʵ�һ�����ܽ���B��N֮��ĵڶ����ڵ�Ԫ����Be��C��O�����֣�
��2����̬K+���Ӻ�������Ų�ʽΪ��1s22s22p63s23p6��������ߵĹ����3p����������������ͼΪ�����Σ�
��3��BeCl2��˫�۷��ӽṹʽΪ��![]() ��Beԭ�ӵĵ����Ų�ͼΪ
��Beԭ�ӵĵ����Ų�ͼΪ![]() ��
��
��4��������BF4-������ԭ����Bԭ�ӣ����ӻ��������Ϊsp3���ķ��������д������Ӽ���Na+��BF4-֮�䣩�����ۼ���B��F֮�䣩����λ����B��F֮�䣬����Bԭ���ṩ�չ��������ѡc��d��e��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�