��Ŀ����
����Ŀ����ش��������⣺
��1����֪2mol����ȼ������Һ̬ˮʱ�ų�572kJ����������Ӧ����ʽ��2H2(g)+O2(g)=2H2O(l)��
�ٸ÷�Ӧ�������������ܺ�__(��������������С��������������)��Ӧ�������ܺ͡�
����2mol������ȫȼ������ˮ��������ų�������___(��������������С��������������)572 kJ��
��2��2.3g�л���C2H6O��һ������������ϵ�ȼ��ǡ����ȫȼ�գ�����CO2��Һ̬ˮ�����ų�68.35kJ��������÷�Ӧ���Ȼ�ѧ����ʽ��___��
��3��FeS2���ղ�����SO2�����������ᡣ��֪25�桢101kPaʱ��
2SO2(g)+O2(g)![]() 2SO3(g) ��H1=-197 kJ��mol-1
2SO3(g) ��H1=-197 kJ��mol-1
H2O(g)=H2O(l) ��H2=-44kJ��mol-1
2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l) ��H3=-545kJ��mol-1
��SO3(g)��H2O(l)��Ӧ����H2SO4(l)���Ȼ�ѧ����ʽ��___��
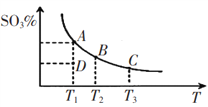
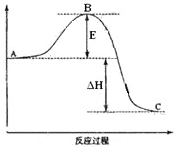
��4��2SO2(g)+O2(g)![]() 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��___(����������������������)����H___(���������������С������������)��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��___(����������������������)����H___(���������������С������������)��
��5���к��Ȳⶨ��ʵ���У��õ��IJ����������ձ����¶ȼơ�___����Ͳ����ȡ��Ӧ��ʱ��ȡ50 mL 0.50 mol��L-1�����ᣬ���������Լ���___(�����)��
A.50 mL0.50mol��L-1NaOH��Һ
B.50 mL0.55mol��L-1NaOH��Һ
C.1.0gNaOH����
���𰸡�С�� С�� C2H6O(l��+3O2��g��=2CO2��g��+3H2O��l) ��H= -1367kJ��mol-1 SO3(g)+H2O(l)=H2SO4(l) ��H= -130kJ��mol-1 ���� ���� ���β�������� B
��������
(1)�ٸ��ݷ��ȷ�Ӧ���������������ͷ�Ӧ���������Ĺ�ϵ�жϣ��ڸ���Һ̬ˮ���ˮ������Ҫ�������жϣ�
(2)���2.3g�л���C2H6O�����ʵ�����Ȼ����������Ȼ�ѧ����ʽ�У���Ӧ��ļ������ͷ�Ӧ�ȳ���������д�Ȼ�ѧ����ʽ��
(3)���ݸ�˹���ɷ������
(4)EΪ��ܣ������ܹ����ͷ�Ӧ�Ļ�ܣ���Ӱ�췴Ӧ��������������ߵͣ��ݴ˷����жϣ�
(5)�����к��ȵIJⶨʵ���õ������������жϣ�Ϊ�˱�֤�����е�һ����ȫ��Ӧ��������֤һ���������ݴ˷����жϡ�
(1)��2H2(g)+O2(g)�T2H2O(l)��H=-572kJ/mol�Ƿ��ȷ�Ӧ�����ȷ�Ӧ�������������������Ӧ������������ʴ�Ϊ��С�ڣ�
�ڸ����Ȼ�ѧ����ʽ2H2(g)+O2(g)�T2H2O(l)��H=-572kJ/mol��2mol������ȫȼ������Һ̬ˮ�ų�����572kJ����Һ̬ˮ���ˮ������Ҫ���ȣ�����2mol������ȫȼ������ˮ�����ų�������С��572kJ���ʴ�Ϊ��С�ڣ�
(2)2.3gC2H6O�����ʵ���Ϊ![]() =0.05mol��ǡ����ȫȼ�գ�����CO2��Һ̬ˮ���ų�68.35kJ��������1mol������ȼ�շ���68.35kJ��
=0.05mol��ǡ����ȫȼ�գ�����CO2��Һ̬ˮ���ų�68.35kJ��������1mol������ȼ�շ���68.35kJ��![]() =1367kJ���Ȼ�ѧ����ʽΪ��C2H6O(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=-1367kJ/mol���ʴ�Ϊ��C2H6O(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=-1367kJ/mol��
=1367kJ���Ȼ�ѧ����ʽΪ��C2H6O(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=-1367kJ/mol���ʴ�Ϊ��C2H6O(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=-1367kJ/mol��
(3)��2SO2(g)+O2(g)2SO3(g)��H1=һ197kJ/mol����H2O (g)=H2O(1)��H2=-44kJ/mol����2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l)��H3=һ545kJ/mol�����ݸ�˹���ɣ�(��-��-2����)��![]() �ã�SO3 (g)+H2O(l)=H2SO4(l) ��H=-130kJ/mol���ʴ�Ϊ��SO3(g)+H2O(l)=H2SO4(l) ��H=-130kJ/mol��
�ã�SO3 (g)+H2O(l)=H2SO4(l) ��H=-130kJ/mol���ʴ�Ϊ��SO3(g)+H2O(l)=H2SO4(l) ��H=-130kJ/mol��
(4)EΪ��ܣ������ܹ����ͷ�Ӧ�Ļ�ܣ���V2O5��ʹͼ��B�㽵�ͣ�����Ӱ�췴Ӧ���������������ߵͣ������H���䣬�ʴ�Ϊ�����ͣ����䣻
(5)�к��ȵIJⶨʵ���õ��IJ��������У��ձ����¶ȼơ���Ͳ�����β����������Ϊ�˱�֤�����е�һ����ȫ��Ӧ��������֤һ����������ȡ��Ӧ��ʱ��ȡ50 mL 0.50 mol��L-1�����ᣬ����ѡ��50mL0.55molL-1NaOH��Һ���ʴ�Ϊ�����β����������B��

 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�