��Ŀ����
����Ŀ������������AC2(����)��B2C2��AD4��Ԫ��A��������ۺ��۾���ֵ���;Ԫ��B�ĵ�������C����̬�����о���ȼ��,����ʻ�ɫ,�����ɵ���ɫ����B2C2;Ԫ��D�ĸ�һ�������ӵ��Ӳ�ṹ���ԭ����ͬ,��:
(1)AC2��AD4�Ļ�ѧʽ�ֱ�Ϊ______��______��
(2)AD4�������к��е���������Ϊ_______(����s-s ��������s-p ����������p-p ������)��
(3)D�ĸ�һ�������ӵĵ����Ų�ʽΪ_________,B2C2�ĵ���ʽΪ__________,��___________(�������ӻ��������������ۻ�������)��
(4)д��һ����AC��Ϊ�ȵ�����ķ���______________
���𰸡�CO2 CCl4 p-p ���� 1s22s22p63s23p6  ���ӻ����� N2
���ӻ����� N2
��������
Ԫ��B�ĵ�������C����̬�����о���ȼ�գ�����ʻ�ɫ�������ɵ���ɫ����B2C2����BΪNa��CΪOԪ�أ�AC2�����壬Ԫ��A��������ۺ��۾���ֵ��ȣ���AΪ�ڢ�A��Ԫ�أ�����AΪ̼Ԫ�أ�Ԫ��D�ĸ�һ�������ӵ��Ӳ�ṹ���ԭ����ͬ����DΪ��Ԫ�أ��ݴ˷�����
Ԫ��B�ĵ�������C����̬�����о���ȼ�գ�����ʻ�ɫ�������ɵ���ɫ����B2C2����BΪNa��CΪOԪ�أ�AC2�����壬Ԫ��A��������ۺ��۾���ֵ��ȣ���AΪ�ڢ�A��Ԫ�أ�����AΪ̼Ԫ�أ�Ԫ��D�ĸ�һ�������ӵ��Ӳ�ṹ���ԭ����ͬ����DΪ��Ԫ�ء�
(1)��������������֪AΪ̼Ԫ�ء�CΪ��Ԫ�ء�DΪ��Ԫ�أ���AC2��AD4�Ļ�ѧʽ�ֱ�ΪCO2��CCl4��
��2��AD4����ΪCCl4������C��Clԭ�ӵ��������Ӷ���p����ϣ����Ժ��е���������Ϊp-p������
(3) ��3�������ӵĵ����Ų�ʽΪ1s22s22p63s23p6 ��B2C2Ϊ�������ƣ������ʽΪ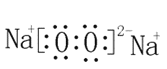 �������ӻ����
�������ӻ����
(4)ACΪCO���ȵ�������ָ�۵�������ԭ�����������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ�ԭ���ţ���CO��Ϊ�ȵ�����ķ�����N2��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ��ũҵ�Ի��ʵ������Ǻϳɰ���ҵ��չ�ij־��ƶ�������һ�ݻ�Ϊ2 L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ��
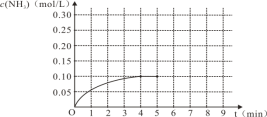
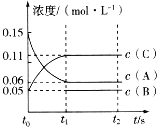
(1)N2(g)��3H2(g)![]() 2NH3(g)����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ������ͼ����ӷ�Ӧ��ʼ��ƽ��ʱ��������ƽ����Ӧ����Ϊ___________________��
2NH3(g)����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ������ͼ����ӷ�Ӧ��ʼ��ƽ��ʱ��������ƽ����Ӧ����Ϊ___________________��
(2)���¶��£���ӦN2(g)+3H2(g)![]() 2NH3(g)+ Q��Q>0����ƽ�ⳣ������ʽΪ__________��
2NH3(g)+ Q��Q>0����ƽ�ⳣ������ʽΪ__________��
��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
T/�� | 25 | 125 | 225 |
ƽ�ⳣ��K | 4��106 | K1 | K2 |
���ж�K1______ K2����д��>����=������<������ԭ����_________________________________
(3)������˵���ϳɰ���Ӧ�Ѵﵽƽ��״̬����________������ĸ������Ӧ���ڹ̶�������ܱ������н��еģ�
a��3v(N2) = v(H2) b�� ![]() �������仯 c�����������ܶȱ��ֲ���
�������仯 c�����������ܶȱ��ֲ���
d��25��ʱ����������� c(NH3)=0.2 mol��L-1�� c(H2) =c(N2) =0.01 mol��L-1
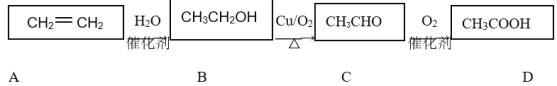
(4) ���������£�NH3����������NO����Ⱦ���������ֶԻ����������ʡ�д����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��____________���÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ____��
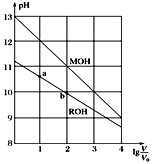
(5) pH��ͬ�İ�ˮ������������Һ���ֱ�������ˮϡ����ԭ�������m����n����ϡ�ͺ�����Һ��pH����ͬ����m________n������>������<������������
����Ŀ����֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ������(������������ȫ������)��

ʵ��ǰ | ʵ��� | |
(�������U�ι�)������ | 101.1g | 102.9g |
(����ʯ��ˮ�����ƿ)������ | 312.0g | 314.2g |
����ʵ��������գ�
��1��ʵ����Ϻ���������ˮ������Ϊ____g��������ƿ������һ�����Σ�������Ϊ______g��
��2�����ɵ�ˮ����Ԫ�ص�����Ϊ________g��
��3�����ɵ�CO2��̼Ԫ�ص�����Ϊ________g��
��4����ȼ����̼����Ԫ��������Ϊ________��