��Ŀ����
��1��д���������ʵĵ��뷽��ʽ��
Na2CO3
��2��322g Na2SO4?10H2O�����ʵ�����
��3����״����33.6LCH4�������ʵ���Ϊ
��4����״���£�ij������ܶ�Ϊ1.96g/L���������ڱ���µ���Է�������Ϊ
Na2CO3
Na2CO3=2Na++CO32-
Na2CO3=2Na++CO32-
��Al2��SO4��3Al2��SO4��3�T2Al3++3SO42-
Al2��SO4��3�T2Al3++3SO42-
����2��322g Na2SO4?10H2O�����ʵ�����
1mol
1mol
������������Na+�����ʵ�����2mol
2mol
������H2O���ӵ���Ŀ��10NA����6.02��1024��
10NA����6.02��1024��
������322gNa2SO4?10H2O����ˮ���2L��Һ�������ʵ���Ũ����0.5mol/L
0.5mol/L
����3����״����33.6LCH4�������ʵ���Ϊ
1.5mol
1.5mol
�������������ʵ���Ϊ15mol
15mol
����4����״���£�ij������ܶ�Ϊ1.96g/L���������ڱ���µ���Է�������Ϊ
44
44
����������1������ǿ�������ȫ���룬������ʲ��ֵ��룬�����Ų����ٲ���з�����
��2������n=
=
������ʵ���ɼ��������������ע��Ħ����������ֵ�ϵ�����Է����������������ʵ���Ũ�ȵļ��㹫ʽ�������Һ��Ũ�ȣ�
��3�����ݱ���µ�����Ħ������������������ʵ��������еĵ�������
��4������������22.4L��������������Ϳ���֪���������Ħ����������Է���������
��2������n=
| m |
| M |
| N |
| NA |
��3�����ݱ���µ�����Ħ������������������ʵ��������еĵ�������
��4������������22.4L��������������Ϳ���֪���������Ħ����������Է���������
����⣺��1��̼��������ǿ����ʣ����뷽��ʽΪ��Na2CO3=2Na++CO32-��
��������ǿ����ʣ�����ȫ���룬���뷽��ʽΪ��Al2��SO4��3�T2Al3++3SO42-��
�ʴ�Ϊ��Na2CO3=2Na++CO32-��Al2��SO4��3�T2Al3++3SO42-��
��2��M��Na2SO4?10H2O��=322g/mol��n��Na2SO4?10H2O��=
=1mol��
n��Na+��=2n��Na2SO4?10H2O��=2��1mol=2mol��
n��H2O��=10n��Na2SO4?10H2O��=10��1mol=10mol��N��H2O��=10mol��NA��/mol=10NA����6.02��1024����
c��Na2SO4��=
=
=0.5mol/L��
�ʴ�Ϊ��1mol��2mol��10NA����6.02��1024����0.5mol/L��
��3������£�33.6L��������ʵ���Ϊ��
=1.5mol�����еĵ��ӵ����ʵ���Ϊ��1.5mol��10=15mol��
�ʴ�Ϊ��1.5mol��15mol��
��4������£�Ħ������Ϊ1.96g/L��22.4L/mol��44g/mol��������Է�������Ϊ44���ʴ�Ϊ��44��
��������ǿ����ʣ�����ȫ���룬���뷽��ʽΪ��Al2��SO4��3�T2Al3++3SO42-��
�ʴ�Ϊ��Na2CO3=2Na++CO32-��Al2��SO4��3�T2Al3++3SO42-��
��2��M��Na2SO4?10H2O��=322g/mol��n��Na2SO4?10H2O��=
| 322g |
| 322g/mol |
n��Na+��=2n��Na2SO4?10H2O��=2��1mol=2mol��
n��H2O��=10n��Na2SO4?10H2O��=10��1mol=10mol��N��H2O��=10mol��NA��/mol=10NA����6.02��1024����
c��Na2SO4��=
| n |
| V |
| 1mol |
| 2L |
�ʴ�Ϊ��1mol��2mol��10NA����6.02��1024����0.5mol/L��
��3������£�33.6L��������ʵ���Ϊ��
| 33.6L |
| 22.4L/mol |
�ʴ�Ϊ��1.5mol��15mol��
��4������£�Ħ������Ϊ1.96g/L��22.4L/mol��44g/mol��������Է�������Ϊ44���ʴ�Ϊ��44��
���������⿼�����й����ʵ����ļ��㣬��Ҫ�����������ʵ�������������ת����ϵ�������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
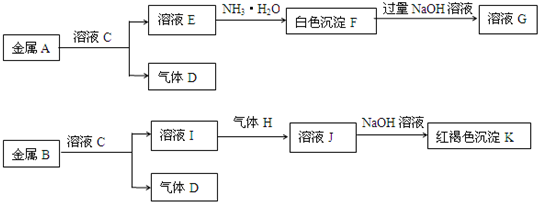
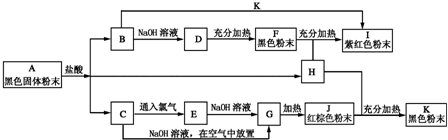
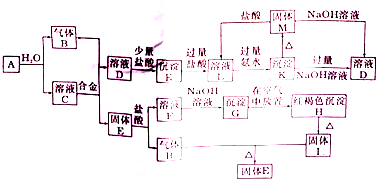
 ��ͼ���������ʵ��ת����ϵ����֪E�ǹ�̬���ʣ������ڻ�ɽ�ڴ������������о����й���E��Ԫ�أ�
��ͼ���������ʵ��ת����ϵ����֪E�ǹ�̬���ʣ������ڻ�ɽ�ڴ������������о����й���E��Ԫ�أ�