Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ≥£ő¬Ō¬£¨ŌÚ20ml£¨0.1mol°§L-1CH3COOH»‹“ļ÷–Ķőľ”0.1mol°§L-1ĶńNaOH»‹“ļ£¨∆špHĪšĽĮ«ķŌŖ»ÁÕľňý ĺ(ļŲ ”ő¬∂»ĪšĽĮ)°£Ō¬Ń–ňĶ∑®÷–īŪőůĶń «

A. cĶ„ĪŪ ĺCH3COOHļÕNaOH«°ļ√∑ī”¶ÕÍ»ę
B. aĶ„ĪŪ ĺĶń»‹“ļ÷–”…ňģĶÁņŽ≥ŲĶńH£ęŇ®∂»ő™1.0°Ń10£≠11mol°§L£≠1
C. bĶ„ĪŪ ĺĶń»‹“ļ÷–c(CH3COO£≠)>c(Na£ę)
D. b°ĘdĶ„ĪŪ ĺĶń»‹“ļ÷–[c(CH3COO-)c(H+)]/c(CH3COOH)ŌŗĶ»
°ĺīūįł°ŅA
°ĺĹ‚őŲ°Ņ
A.”…÷–ļÕ∑ī”¶CH3COOH+NaOH=CH3COONa+H2OŅ…÷™£¨CH3COOHļÕNaOH«°ļ√∑ī”¶ÕÍ»ę Ī»‹“ļő™CH3COONa»‹“ļ£¨∂Ýł√«ŅľÓ»űňŠ—ő»‹“ļ÷–īś‘ŕňģĹ‚∆Ĺļ‚CH3COO-+H2O![]() CH3COOH+OH-£¨»‹“ļŌ‘ľÓ–‘£¨∂ÝcĶ„»‹“ļŌ‘÷––‘£¨ňý“‘cĶ„≤Ľń‹ĪŪ ĺCH3COOHļÕNaOH«°ļ√∑ī”¶ÕÍ»ę£¨AŌÓīŪőů£Ľ
CH3COOH+OH-£¨»‹“ļŌ‘ľÓ–‘£¨∂ÝcĶ„»‹“ļŌ‘÷––‘£¨ňý“‘cĶ„≤Ľń‹ĪŪ ĺCH3COOHļÕNaOH«°ļ√∑ī”¶ÕÍ»ę£¨AŌÓīŪőů£Ľ
B.aĶ„ĪŪ ĺĶő∂®«įĶńī◊ňŠ»‹“ļ£¨łýĺ›pH=3÷™»‹“ļ÷–H+Ň®∂»ő™10-3mol/L£¨‘Ú»‹“ļ÷–OH-Ň®∂»=![]() mol/L£¨ī◊ňŠ»‹“ļ÷–īś‘ŕňģĶńĶÁņŽH2O
mol/L£¨ī◊ňŠ»‹“ļ÷–īś‘ŕňģĶńĶÁņŽH2O![]() H++OH-£¨ł√ĶÁņŽ∆Ĺļ‚ ‹ĶĹī◊ňŠĶń“÷÷∆◊ų”√£¨ł√»‹“ļ÷–ĶńOH-»ę≤Ņ”…ňģĶÁņŽ≤ķ…ķ£¨ňý“‘ňģĶÁņŽ≥ŲĶńH+Ň®∂»=ňģĶÁņŽ≥ŲĶńOH-»‹“ļ=10-11mol/L£¨BŌÓ’ż»∑£Ľ
H++OH-£¨ł√ĶÁņŽ∆Ĺļ‚ ‹ĶĹī◊ňŠĶń“÷÷∆◊ų”√£¨ł√»‹“ļ÷–ĶńOH-»ę≤Ņ”…ňģĶÁņŽ≤ķ…ķ£¨ňý“‘ňģĶÁņŽ≥ŲĶńH+Ň®∂»=ňģĶÁņŽ≥ŲĶńOH-»‹“ļ=10-11mol/L£¨BŌÓ’ż»∑£Ľ
C.”…ÕľŌŮŅ…÷™bĶ„»‹“ļ÷–≥¨Ļż“ĽįŽĶńī◊ňŠĪĽ÷–ļÕ£¨»‹÷ «CH3COONaļÕCH3COOHĶńĽžļŌ»‹“ļ£¨»‹“ļŌ‘ňŠ–‘ľīc(H+)>c(OH-)£¨łýĺ›ĶÁļ… ōļ„”–£ļc(CH3COO-)+c(OH-)=c(Na+)+c(H+)£¨“Úīňc(CH3COO-)>c(Na+)£¨CŌÓ’ż»∑£Ľ
D.‘ŕĶő∂®Ļż≥Ő÷–£®įŁņ®b°ĘdŃĹĶ„£©∂ľ”–ī◊ňŠĶńĶÁņŽ∆Ĺļ‚Ķń“∆∂Į£ļCH3COOH![]() CH3COO-+H+£¨∆šĶÁņŽ∆Ĺļ‚≥£ żő™
CH3COO-+H+£¨∆šĶÁņŽ∆Ĺļ‚≥£ żő™![]() £¨∂ÝK÷Ľ”Žő¬∂»”–Ļō£¨”Žī◊ňŠĶńŇ®∂»őřĻō£¨ňý“‘b°ĘdĶ„ĪŪ ĺĶń»‹“ļ÷–
£¨∂ÝK÷Ľ”Žő¬∂»”–Ļō£¨”Žī◊ňŠĶńŇ®∂»őřĻō£¨ňý“‘b°ĘdĶ„ĪŪ ĺĶń»‹“ļ÷–![]() ÷ĶŌŗĶ»£¨DŌÓ’ż»∑£Ľīūįł—°A°£
÷ĶŌŗĶ»£¨DŌÓ’ż»∑£Ľīūįł—°A°£

 ĺŔ“Ľ∑ī»żÕ¨≤Ĺ«…Ĺ≤ĺęŃ∑ŌĶŃ–īūįł
ĺŔ“Ľ∑ī»żÕ¨≤Ĺ«…Ĺ≤ĺęŃ∑ŌĶŃ–īūįł Ņŕň„”Ž”¶”√Ő‚Ņ®ŌĶŃ–īūįł
Ņŕň„”Ž”¶”√Ő‚Ņ®ŌĶŃ–īūįł°ĺŐ‚ńŅ°ŅĻż—űĽĮ«‚(H2O2) «÷ō“™ĶńĽĮĻ§≤ķ∆∑£¨Ļ„∑ļ”¶”√”ŕ¬Ő…ęĽĮ—ßļŌ≥…£ģ“ĹŃ∆ŌŻ∂ĺĶ»Ńž”Ú°£
ĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©“—÷™£ļH2(g)£ę![]() O2(g)£ĹH2O(l) °ųH1£Ĺ£≠286 kJ°§mol1
O2(g)£ĹH2O(l) °ųH1£Ĺ£≠286 kJ°§mol1
H2(g)£ęO2(g)£ĹH2O2(l) °ųH2£Ĺ£≠188 kJ°§mol1
Ļż—űĽĮ«‚∑÷Ĺ‚∑ī”¶2H2O2(l)£Ĺ2H2O(l)£ęO2(g)Ķń°ųH£Ĺ______kJ°§mol1°£≤ĽÕ¨ő¬∂»Ō¬Ļż—űĽĮ«‚∑÷Ĺ‚∑ī”¶Ķń∆Ĺļ‚≥£ żK(313K)_____K(298K) (ŐÓīů”ŕ°Ę–°”ŕĽÚĶ»”ŕ)°£
£®2£©100°ś Ī£¨‘ŕ≤ĽÕ¨Ĺū ŰņŽ◊”īś‘ŕŌ¬£¨īŅĻż—űĽĮ«‚24 hĶń∑÷Ĺ‚¬ ľŻŌ¬ĪŪ£ļ
ņŽ◊” | ľ”»ŽŃŅ(mg°§L1) | ∑÷Ĺ‚¬ % | ņŽ◊” | ľ”»ŽŃŅ(mg°§L1) | ∑÷Ĺ‚¬ £• |
őř | 0 | 2 | Fe3£ę | 1.0 | 15 |
Al3£ę | 10 | 2 | Cu2£ę | 0.1 | 86 |
Zn2£ę | 10 | 10 | Cr3£ę | 0.1 | 96 |
”……ŌĪŪ żĺ›Ņ…÷™£¨ń‹ ĻĻż—űĽĮ«‚∑÷Ĺ‚∑ī”¶ĽÓĽĮń‹ĹĶĶÕ◊Ó∂ŗĶńņŽ◊” «_______°£÷Ł‘ňĻż—űĽĮ«‚ Ī£¨Ņ…—°”√Ķń»›∆ų≤ń÷ ő™_________(ŐÓĪÍļŇ)°£
A£ģ≤Ľ–‚ł÷ B£ģīŅ¬Ń C£ģĽ∆Õ≠ D£ģ÷żŐķ
£®3£©Ļż—űĽĮ«‚ĶńKa1£Ĺ2.24°Ń1012£¨H2O2ĶńňŠ–‘________H2O (ŐÓīů”ŕ°Ę–°”ŕĽÚĶ»”ŕ)°£
—–ĺŅĪŪ√ų£¨Ļż—űĽĮ«‚»‹“ļ÷–HO2-ĶńŇ®∂»‘Ĺīů£¨Ļż—űĽĮ«‚Ķń∑÷Ĺ‚ňŔ¬ ‘ĹŅž°£≥£ő¬Ō¬£¨≤ĽÕ¨Ň®∂»ĶńĻż—űĽĮ«‚∑÷Ĺ‚¬ ”ŽpHĶńĻōŌĶ»ÁÕľňý ĺ°£“Ľ∂®Ň®∂»ĶńĻż—űĽĮ«‚£¨pH‘Ųīů∑÷Ĺ‚¬ ‘ŲīůĶń‘≠“Ú «___________________£ļŌŗÕ¨pHŌ¬£¨Ļż—űĽĮ«‚Ň®∂»‘Ĺīů∑÷Ĺ‚¬ ‘ĹĶÕĶń‘≠“Ú «__________________________________________°£

°ĺŐ‚ńŅ°ŅŌ¬Ń– Ķ—ť≤Ŕ◊ų∂‘”¶Ķń Ķ—ťŌ÷ŌůľįĹ‚ ÕĽÚĹŠ¬Ř∂ľ’ż»∑Ķń «(°°°°)
—°ŌÓ | Ķ—ť≤Ŕ◊ų | Ķ—ťŌ÷Ōů | Ĺ‚ ÕĽÚĹŠ¬Ř |
A | ŌÚFe(NO3)2»‹“ļ÷–Ķő»ŽŃÚňŠňŠĽĮĶńH2O2»‹“ļ | »‹“ļĪšő™Ľ∆…ę | —űĽĮ–‘£ļH2O2>Fe3£ę |
B | ŌÚ5mL1mol/L NaOH»‹“ļ÷–Ķőľ”5Ķő1mol/L MgCl2»‹“ļ£¨»Ľļů‘ŔĶőľ”◊„ŃŅĶń1mol/L CuCl2»‹“ļ | Ō»≤ķ…ķį◊…ę≥ŃĶŪ£¨»Ľļů≤ķ…ķņ∂…ę≥ŃĶŪ | Ksp[Cu(OH)2] >Ksp[Mg(OH)2] |
C | Ĺę≥š¬ķNO2Ķń√‹Ī’≤£Ńß«ÚĹĢŇ›‘ŕ»»ňģ÷– | ļž◊ō…ęĪš…Ó | 2NO2(g) |
D | ŌÚ“Ľ∂®ŃŅňŠ–‘KMnO4»‹“ļ÷–ľ”»Ž““∂Ģīľ£®HOCH2CH2OH£© | »‹“ļ◊Ō…ęÕ »• | ““∂ĢīľĪĽ—űĽĮő™““∂ĢňŠ |
A. A B. B C. C D. D
°ĺŐ‚ńŅ°ŅŇū√ĺńŗ «“Ľ÷÷Ļ§“Ķ∑ŌŃŌ£¨÷ų“™≥…∑› «MgO£®’ľ40%£¨÷ ŃŅ∑÷ ż£©£¨ĽĻ”–CaO°ĘMnO°ĘFe2O3°ĘFeO°ĘAl2O3°ĘSiO2Ķ»‘”÷ £¨“‘īňő™‘≠ŃŌ÷∆»°ĶńŃÚňŠ√ĺ£¨Ņ…”√”ŕ”°»ĺ°Ę‘ž÷Ĺ°Ę“Ĺ“©Ķ»Ļ§“Ķ°£ī”Ňū√ĺńŗ÷–ŐŠ»°MgSO47H2OĶńĻ§“’Ńų≥Ő»ÁŌ¬£ļ
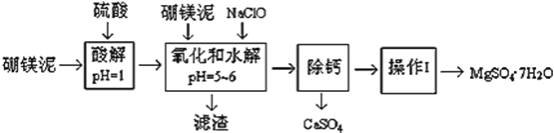
£®1£© Ķ—ť÷––Ť“™1 mol/LĶńŃÚňŠ800 mL£¨»Ű”√ 98% ĶńŇ®ŃÚňŠ£®¶—= 1.84 g/mL£©ņīŇš÷∆£¨ŃŅ»°Ň®ŃÚňŠ–Ť“™ Ļ”√ŃŅÕ≤ĶńĻśłŮő™__________£®ŐÓ–ī—°ŌÓ◊÷ńł£©
A£ģ10 mL B£ģ20 mL C£ģ50 mL D£ģ100 mL
£®2£©ľ”»ŽĶńNaClOŅ…”ŽMn2+ ∑ī”¶£ļMn2+ + ClO + H2O = MnO2°ż+ 2H+ + Cl£¨‘ŕł√≤Ĺ÷Ť÷–ĽĻ”–“Ľ÷÷ņŽ◊”“≤ĽŠĪĽNaClO—űĽĮ£¨ł√∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺő™___________________°£
£®3£©¬ň‘ŁĶń÷ų“™≥…∑÷≥żļ¨”–Fe(OH)3°ĘAl(OH)3Õ‚£¨ĽĻļ¨”–__________________°£
£®4£©‘ŕ°į≥żł∆°Ī«į£¨–Ťľž—ť¬ň“ļ÷–Fe3+ «∑ŮĪĽ≥żĺ°£¨ľÚ Ųľž—ť∑Ĺ∑®___________________°££®–ī≥Ų≤Ŕ◊ų°ĘŌ÷ŌůļÕĹŠ¬Ř£©
£®5£©“—÷™MgSO4°ĘCaSO4 Ķń»‹Ĺ‚∂»£®Ķ•őĽő™ g/100 g ňģ£©»ÁŌ¬ĪŪ£ļ
ő¬∂»£®°ś£© | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
°į≥żł∆°Ī «ĹęMgSO4ļÕCaSO4ĽžļŌ»‹“ļ÷–ĶńCaSO4≥ż»•£¨łýĺ›…ŌĪŪ żĺ›£¨ľÚ“™ňĶ√ų≤Ŕ◊ų≤Ĺ÷Ť_____________________________°£°į≤Ŕ◊ųĘŮ°Ī «Ĺę¬ň“ļľŐ–Ý’Ű∑ĘŇ®ňű£¨ņš»īĹŠĺߣ¨______£¨Ī„Ķ√ĶĹŃňMgSO47H2O .
°ĺŐ‚ńŅ°Ņt °ś Ī£¨‘ŕŐŚĽż≤ĽĪšĶń√‹Ī’»›∆ų÷–∑Ę…ķ∑ī”¶£ļX(g)£ę3Y(g)![]() 2Z(g)£¨łų◊ť∑÷‘ŕ≤ĽÕ¨ ĪŅŐĶńŇ®∂»»ÁŌ¬ĪŪ£ļ
2Z(g)£¨łų◊ť∑÷‘ŕ≤ĽÕ¨ ĪŅŐĶńŇ®∂»»ÁŌ¬ĪŪ£ļ
őÔ÷ | X | Y | Z |
≥ű ľŇ®∂»/(mol°§L£≠1) | 0.1 | 0.2 | 0 |
2 minń©Ň®∂»/(mol°§L£≠1) | 0.08 | a | b |
∆Ĺļ‚Ň®∂»/(mol°§L£≠1) | 0.05 | 0.05 | 0.1 |
Ō¬Ń–ňĶ∑®’ż»∑Ķń «(°°°°)
A. ∆Ĺļ‚ Ī£¨XĶń◊™ĽĮ¬ ő™20%
B. 2 min ĪYĶńőÔ÷ ĶńŃŅő™0.14
C. ‘Ųīů∆Ĺļ‚ļůĶńŐŚŌĶ—Ļ«Ņ£¨v(’ż)‘Ųīů£¨v(ńś)ľű–°
D. 2 minńŕ£¨”√YĶńĪšĽĮŃŅĪŪ ĺĶń∆Ĺĺý∑ī”¶ňŔ¬ v(Y)£Ĺ0.03 mol°§L®D1°§min®D1