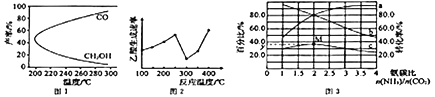
��Ŀ����
����Ŀ��ijУ����ѧϰС���ͬѧ���ʵ����֤Na2SO4�뽹̿���¼��Ⱥ�IJ���ش��������⡣
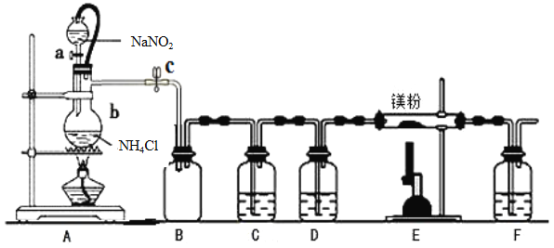
(1)Na2SO4�뽹̿��Ӧ��ʵ��װ������ͼ��ʾ��
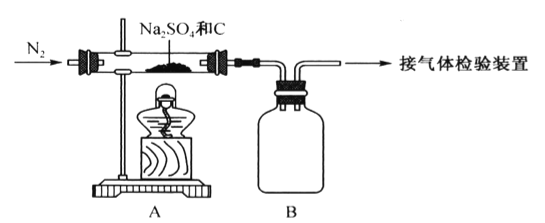
����ͨ��N2,Ȼ����ȣ�ֱ����Ӧ����������������N2��������__________________________��
��װ��B��������___________��
(2)��ͬѧ��Ϊ��������п��ܺ���CO2��CO ��SO2����������֤��ѡ������ʵ���е�װ��A��B����ͼ��ʾ�IJ���װ��(�����ظ�ѡ��)����ʵ�顣
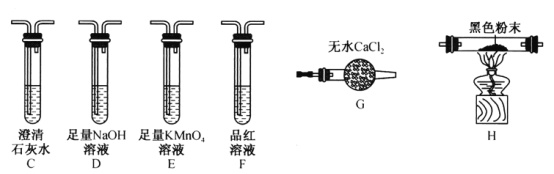
��ʵ��װ�����ӵĺ���˳��ΪA��B��___________��װ��H�к�ɫ��ĩ��___________��
����֤����������CO��������____________________________________________��
��������SO2��Eװ�õ�������______________________________(�û�ѧ����ʽ˵��)��
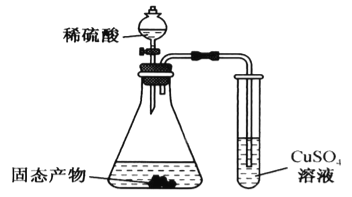
(3)ijͬѧ������ͼװ����֤��̬����,��֤����̬�����к���Na2S��������___________����ʵ���з��ֹ�̬������ȫ��Ӧ����ƿ�ײ�������������ɫ������˵����������г�����Na2S��,������������___________(��һ�ֿ��ܵ�����)��
���𰸡� �ų�װ���п����������ɵ����崵�� ��ȫƿ FECDGHC CuO H�к�ɫ��ĩ��Ϊ��ɫ������C�г���ʯ��ˮ����� 5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4 �Թ����к�ɫ���� Na2SO3
��������(1)��̼�ܹ�������е�������Ӧ����̼�������Ϊ�˷�ֹ������ʵ��ĸ��ţ���ͨ��N2��Ȼ����ȣ�ֱ����Ӧ�����������ų�װ���п����������ɵ����崵�����ʴ�Ϊ���ų�װ���п����������ɵ����崵����
��Na2SO4�뽹̿���¼��Ⱥ�IJ����п��ܺ��ж��������������ˮ�����壬װ��B���Է�ֹ���������������������ɵĵ������ʴ�Ϊ����ȫƿ(���ֹ����)��
(2)�������п��ܺ���CO2��CO��SO2�����ڶ�������Ҳ��ʹ�����ʯ��ˮ����ǣ���Ҫ���ȼ����������Ȼ���ȥ����������ټ��������̼������ȥ������̼�����һ����̼��ʵ��װ�����ӵĺ���˳��ΪA��B��F��E��C��D��G��H��C��һ����̼���л�ԭ�ԣ�����ʹ���������ﻹԭ��װ��H�к�ɫ��ĩ����������ͭ���ʴ�Ϊ��FECDGHC��CuO��
��H�к�ɫ��ĩ��Ϊ��ɫ������C�г���ʯ��ˮ����ǣ�����֤����������CO���ʴ�Ϊ��H�к�ɫ��ĩ��Ϊ��ɫ������C�г���ʯ��ˮ�������
�۶��������ܹ�ʹ���������Һ��ɫ����Ӧ�Ļ�ѧ����ʽΪ5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4���ʴ�Ϊ��5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4��
(3) Na2S�ܹ������ᷴӦ�����������壬����������ͭ��Һ��Ӧ���ɺ�ɫ����ͭ������ʵ���з��ֹ�̬������ȫ��Ӧ����ƿ�ײ�������������ɫ�������û�ɫ����Ӧ�����������������Ӧ���ɵ���˵����������г�����Na2S��,������������Na2SO3���ʴ�Ϊ���Թ����к�ɫ������Na2SO3��

����Ŀ����2 L�ܱ������ڣ�800 ��ʱ��Ӧ��2NO(g)��O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��/(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/(mol) | 0.20 | 0.10 | 0.08 | 0.07 | 0.07 | 0.07 |
(1)�ﵽƽ���ʱ����________��������___________________��
(2)��ͼ�б�ʾNO2��Ũ�ȱ仯��������________����O2��ʾ��0��2 s�ڸ÷�Ӧ��ƽ������v��________��

(3)��˵���÷�Ӧ�Ѵﵽƽ��״̬����________��
a��v(NO2)��2v(O2)
b�������ڸ����ʵ�Ũ�ȱ��ֲ���
c��v��(NO)��2v��(O2)
d���ﵽ��ѧƽ��ʱ��NO����ȫת��ΪNO2
(4)������÷�Ӧ�ķ�Ӧ���ʵ���________��
a����ʱ�����NO2����
b���ʵ������¶�
c������O2��Ũ��
d��ѡ���Ч����