��Ŀ����
ͨ�����·�Ӧ���ɻ�ȡH2�������й�˵����ȷ����( )
��̫������ֽ�ˮ���⣺2H2O(l)��2H2(g)+O2(g)��H1=+571.6kJ��mol�C1
�ڽ�̿��ˮ��Ӧ���⣺C(s)+H2O(g)��CO(g)+H2(g)��H2=+131.3kJ��mol�C1
�ۼ�����ˮ��Ӧ���⣺CH4(g)+H2O(g)��CO(g)+3H2(g)��H3=+206.1kJ��mol�C1
A����Ӧ���е���ת��Ϊ��ѧ��
B����Ӧ��Ϊ���ȷ�Ӧ
C����Ӧ��ʹ�ô�������H3��С
D����ӦCH4(g)��C(s)+2H2(g)�Ħ�H=+74.8kJ��mol�C1
��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
12�����������ڴ�������ȼ�գ�ǡ����ȫ��Ӧ�����������ڻ������ǣ�������
| A�� | þ�� | B�� | ���� | C�� | ���� | D�� | ��˿ |
17�����������һ�ָ�Ч��ˮ��������ǿ�����ԣ���ɱ��������������Դ�������ȷ������Ź㷺����;��ʪ�����ɷ��Ʊ��������ε�ԭ�������ʾ��
��1����ҵ����ʪ���Ʊ�������أ�K2FeO4����������ͼ��ʾ��
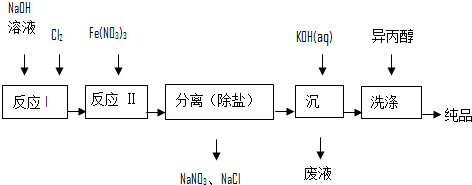
��ϴ�Ӵ�Ʒʱѡ�������������ˮ�������ǣ����ٸ�����ص��ܽ���ʧ��
�ڷ�Ӧ������ӷ���ʽΪCl2+20H-=Cl-+ClO-+H2O��
�۷�Ӧ�����������뻹ԭ��֮��Ϊ3��2��
����֪25��ʱFe��OH��3��Ksp=4.0��10-38����Ӧ������Һ��c��Fe3+��=4.0��10-5 mol/L������Ҫ����pH=3ʱ����ʼ����Fe��OH��3����������Һ����ı仯����
��2��������ͼ�ɼ���ʪ���Ʊ��������ʱ�������Ƶø������ƣ�Ȼ��������������м��뱥��KOH��Һ����������������أ�
�ټ��뱥��KOH��Һ��Ŀ���ǣ�����K+Ũ�ȣ��ٽ�������ؾ���������
����������Ϣ��֪��������ص��ܽ�ȱȸ�������С�����С������
��3����д���ɷ��Ʊ�K2FeO4�Ļ�ѧ��Ӧ����ʽFe2O3+3KNO3+4KOH=2K2FeO4+3KNO2+2H2O��
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��NaClO��Ӧ�����Ϻ�ɫ����������Һ |
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ�������κ�KNO2�Ȳ��� |

��ϴ�Ӵ�Ʒʱѡ�������������ˮ�������ǣ����ٸ�����ص��ܽ���ʧ��
�ڷ�Ӧ������ӷ���ʽΪCl2+20H-=Cl-+ClO-+H2O��
�۷�Ӧ�����������뻹ԭ��֮��Ϊ3��2��
����֪25��ʱFe��OH��3��Ksp=4.0��10-38����Ӧ������Һ��c��Fe3+��=4.0��10-5 mol/L������Ҫ����pH=3ʱ����ʼ����Fe��OH��3����������Һ����ı仯����
��2��������ͼ�ɼ���ʪ���Ʊ��������ʱ�������Ƶø������ƣ�Ȼ��������������м��뱥��KOH��Һ����������������أ�
�ټ��뱥��KOH��Һ��Ŀ���ǣ�����K+Ũ�ȣ��ٽ�������ؾ���������
����������Ϣ��֪��������ص��ܽ�ȱȸ�������С�����С������
��3����д���ɷ��Ʊ�K2FeO4�Ļ�ѧ��Ӧ����ʽFe2O3+3KNO3+4KOH=2K2FeO4+3KNO2+2H2O��
18�� ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��N{O}_{2}��}{{c}^{2}��NO����c��{O}_{2}��}$��
��2����֪��K300����K350������÷�Ӧ�Ƿ����ȷ�Ӧ��
��3����ͼ��ʾNO2�ı仯��������b����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=1.5��10-3mol•L-1•s-1��
��4��Ϊʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����c��
a����ʱ�����NO2b���ʵ�����c������O2��Ũ��d��ѡ�������
 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��2����֪��K300����K350������÷�Ӧ�Ƿ����ȷ�Ӧ��
��3����ͼ��ʾNO2�ı仯��������b����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=1.5��10-3mol•L-1•s-1��
��4��Ϊʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����c��
a����ʱ�����NO2b���ʵ�����c������O2��Ũ��d��ѡ�������


 ��
�� ��������ͬѧ��2mLFeCl2��Һ���ȼ���0.5mLú�ͣ�����Һ�������μ��뼸����ˮ��l��KSCN��Һ����Һ��죬ú�͵�������____ __��
��������ͬѧ��2mLFeCl2��Һ���ȼ���0.5mLú�ͣ�����Һ�������μ��뼸����ˮ��l��KSCN��Һ����Һ��죬ú�͵�������____ __�� SCN��Һ����
SCN��Һ����