��Ŀ����
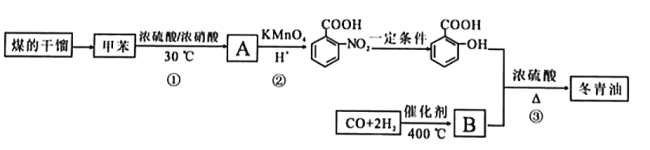
����Ŀ���ױ���ú����IJ���,�������Ʊ�����ֹʹ����Ч���Ķ�����( 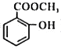 )���ϳ�·�����£�
)���ϳ�·�����£�
��֪��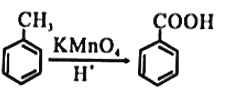
��ش���������
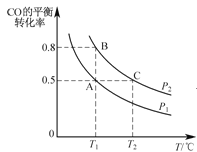
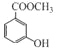
��1��ú�ĸ�����_______________��(���������仯��������ѧ�仯��).
��2��A�Ľṹ��ʽΪ_______________����Ӧ�ٵķ�Ӧ������_______________��
��3����Ӧ�ڵķ�Ӧ������_______________��
��4��B�Ĺ���������Ϊ_______________��
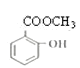
��5����Ӧ�۵Ļ�ѧ����ʽ_______________��
��6��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ����C�Ľṹ��ʽ����Ϊ__________��
���𰸡���ѧ�仯 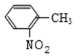 ȡ����Ӧ ������Ӧ �ǻ�
ȡ����Ӧ ������Ӧ �ǻ� ![]() +CH3OH
+CH3OH![]()
 +H2O ��
+H2O ��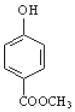 ��
�� ��
��
��������
�ױ���Ũ���ᷢ��ȡ����Ӧ����A��A����������Ӧ���������������ᣬ����������������֪���ױ������ᷢ����λȡ������AΪ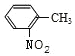 ����������������һ�������·�Ӧ�������ǻ������ᣬB�����ǻ������ᷴӦ����
����������������һ�������·�Ӧ�������ǻ������ᣬB�����ǻ������ᷴӦ���� ����BΪCH3OH�������Ŀ�������
����BΪCH3OH�������Ŀ�������
(1)ú�ĸ�������������������ɣ����Ը����ǻ�ѧ�仯��
�ʴ�Ϊ����ѧ�仯��
(2)A�Ľṹ��ʽΪ ����Ӧ�ٵķ�Ӧ������ȡ����Ӧ��
����Ӧ�ٵķ�Ӧ������ȡ����Ӧ��
�ʴ�Ϊ�� ��ȡ����Ӧ��
��ȡ����Ӧ��
(3)��Ӧ�ڵķ�Ӧ������������Ӧ��
�ʴ�Ϊ��������Ӧ��
(4)BΪCH3OH��B�Ĺ���������Ϊ�ǻ���
�ʴ�Ϊ���ǻ���
(5)��Ӧ�۵Ļ�ѧ����ʽΪ![]() +CH3OH
+CH3OH![]()
 +H2O��
+H2O��
�ʴ�Ϊ��![]() +CH3OH
+CH3OH![]()
 +H2O��
+H2O��
(6)������Ϊ ��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ��ΪCOOCH3��OH����C�Ľṹ��ʽ����Ϊ
��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ��ΪCOOCH3��OH����C�Ľṹ��ʽ����Ϊ ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��

����Ŀ��ij��ȤС���Ʊ�һ��������������.ȡ3mL��ˮ�Ҵ�,2mLŨ����,2mL���������ʵ�飬��5mL����̼������Һ�ռ����
I.ʵ��װ����ͼ��ʾ
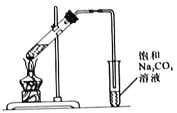
��1���Ʊ����������Ļ�ѧ����ʽΪ_______________��
��2��Ũ�����������_______________��
��3�������ܵ�������_______________��
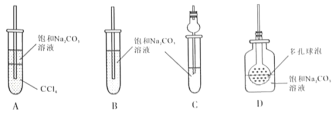
��4������װ�û���ѡ����ͼ�е�___________��(�����).
��.��ͬѧ�ú��з�̪�ı���̼������Һ(�ʼ���)�ռ�����������ֺ�ɫѸ����ȥ.
��ͬѧ��Ϊ�������������к���̼����.��ͬѧͨ���������ϲ���������ʵ�飬֤����ͬѧ���Ʋ��Ǵ���ġ�
��֪����̪������ˮ���������л��ܼ�����̪�Լ��Ƿ�̪���Ҵ���Һ.
ʵ��i��ȡ����²���ɫҺ�壬�ֳ����ݣ��ֱ��������ʵ��
��� | ʵ����� | ʵ������ | ���� |
1 | �μӼ�����̪�Լ� | ��Һ �� (��������������������) | ̼���Ʋ�δ��������ȫ�кͣ����д���ʣ�� |
2 | ����������Һ | �д������ݲ��� |
ʵ��ii.ȡ����ϲ�Һ�壬���� �� ��Һ�������ֳ���dz��ɫ�����÷ֲ���ɫ��ʧ��
ʵ��iii��ȡ5mL����̼������Һ�����뼸�η�̪�Լ����ټ���3mL��������(��������)����Һ�ȱ�죬���ɫ��ʧ���ش���������
��5���������ʵ�飺��_______________����_______________��
��6�����ʵ��ii��ʵ��iii�����ɵó��Ľ�����_______________��
��7��ʵ��iii��ʵ��Ŀ����_______________��