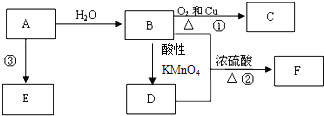
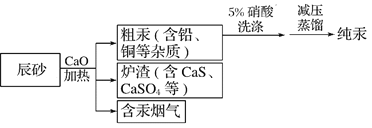
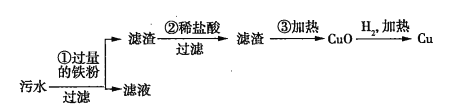
��Ŀ����
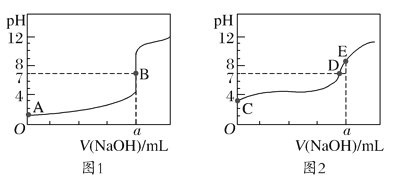
����Ŀ����1����������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ����1L�ܱ������У���ʼͶ��4mol N2��6mol H2��һ������������NH3��ƽ��ʱ���ı��¶Ȳ�õ����������ʾ ����֪��T1<T2��
�¶� | ƽ��ʱNH3�����ʵ���/mol |
T1 | 3.6 |
T2 | 2 |
����K1_______K2 ����������������������=������ԭ��__________��
����T2�£�����10s�ﵽ��ѧƽ��״̬����ƽ��ʱH2��ת����Ϊ______________������ͬʱ���Ӹ����ʵ���Ϊ1mol����÷�Ӧ������v��_____v��������=����ƽ�ⳣ����______������������������С���������䣩��
��2��һ���¶��£���3mol A�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��
3A��g��+B��g��![]() xC��g��������д���пհף�
xC��g��������д���пհף�
�ٷ�Ӧ1minʱ���ʣ��1.8mol A��C��Ũ��Ϊ0.4 mol/L����1min��B�ķ�Ӧ����Ϊ______��xΪ_______��
�������������ʼѹǿΪP0��10min���ƽ�⣬�����ڻ��������ѹǿΪP����P0��P����ʾ��ƽ��ʱ��Ӧ��A��ת����a��A��Ϊ__________��
���ܹ�˵���÷�Ӧ�ﵽƽ��ı�־��___________��
A �����ڻ��������ܶȱ��ֲ���
B v��A��=3v��B��
C A��B��Ũ��֮��Ϊ3:1
D ��λʱ��������3 n molA��ͬʱ����n mol B
E ��ϵ���¶Ȳ��ٱ仯
���𰸡��� ͬһ�����£��¶Ȳ�ͬ����Ӧ���ɲ���Խ�࣬KԽ�� 50% �� ���� 0.2 mol/��L��min�� 2 ![]() D
D
��������
��1����ʼͶ��4mol N2��6mol H2��һ������������NH3������������ʵ���Խ��˵����Ӧ���еij̶�Խ���¶ȸߣ����ʵͣ������ƽ�������ƶ�������ӦΪ���ȷ�Ӧ���ɴ˿��ƶϳ�ƽ�ⳣ�����¶ȵĹ�ϵ������ƽ��ʱn(NH3)��������仯�����Ӷ����ת���ʣ�����ƽ��ʱŨ�ȿ����ƽ�ⳣ��������Ũ������ƽ�ⳣ���Ĺ�ϵ������ȷ��ƽ���ƶ��ķ��Ӷ����v����v����
��2����������ʽ�������v(B)��X������ѹǿ֮�ȵ������ʵ���Ũ��֮�ȣ�������仯�����Ӷ����ת���ʡ�
��1������Ϊ�¶����ߣ�n(NH3)��С�������ƽ�������ƶ���Kֵ��С������K1<K2��ԭ��ͬһ�����£��¶Ȳ�ͬ����Ӧ���ɲ���Խ�࣬KԽ��
��Ϊ��<��ͬһ�����£��¶Ȳ�ͬ����Ӧ���ɲ���Խ�࣬KԽ��
�� 3H2��g��+N2��g��![]() 2NH3��g����
2NH3��g����
��ʼ�� 6mol/L 4mol/L 0
�仯�� 3mol/L 1mol/L 2 mol/L
ƽ���� 3mol/L 3mol/L 2 mol/L
��ƽ��ʱH2��ת����Ϊ![]() ��
��
K=![]() ��Q=
��Q=![]() <0.049������ƽ�������ƶ���
<0.049������ƽ�������ƶ���
��÷�Ӧ������v��>v������Ϊ�¶Ȳ��䣬����ƽ�ⳣ�����䣻
��Ϊ��50%�� �������䡣
��2���� 3A��g�� + B��g��![]() xC��g��
xC��g��
��ʼ�� 1.5mol/L 0.5mol/L 0
�仯�� 0.6mol/L 0.2mol/L 0.4mol/L
ƽ���� 0.9mol/L 0.3mol/L 0.4mol/L
1min��B�ķ�Ӧ����Ϊ![]() mol/��L��min����
mol/��L��min����
���û�ѧ������֮�ȵ���Ũ�ȱ仯��֮�ȣ������x=2��
����. 0.2 mol/��L��min����2��
����A��10min�ڵ�Ũ�ȱ仯��Ϊ3x
3A��g�� + B��g��![]() 2C��g��
2C��g��
��ʼ�� 1.5mol/L 0.5mol/L 0
�仯�� 3x x 2x
ƽ���� 1.5-3x 0.5-x 2x
![]() ��x=1-
��x=1-![]() ��
��
��ƽ��ʱ��Ӧ��A��ת����a��A��Ϊ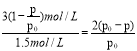 ��
��
����![]() ��
��
��A .��Ϊ������������䡢�������ݻ����䣬���������ڻ��������ܶ�ʼ�ղ��䣬A��һ����ƽ��״̬��
B.��ΪA��B����ʼͶ����֮�ȵ��ڻ�ѧ������֮��3:1������ʼ�մ���v��A��=3v��B����B��һ����ƽ��״̬��
C. ��ΪA��B����ʼͶ����֮�ȵ��ڻ�ѧ������֮��3:1������ʼ�մ���A��B��Ũ��֮��Ϊ3:1��C��һ����ƽ��״̬��
D. ��λʱ��������3 n molA��ͬʱ����n mol B����ֵ֮�ȵ��ڻ�ѧ������֮���ҷ����෴��D��ƽ��״̬��
E. ��Ϊ�Ǻ���������������ϵ���¶�ʼ�ղ��䣬E��һ����ƽ��״̬
����D��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��X��Y��������������Z��Һ�й�����ͼ��װ�ã�ʵ���е�����ָ�뷢��ƫת��ͬʱX����֣�Y����ϸ����X��Y��Z������( )
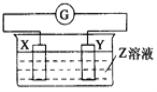
ѡ�� | X | Y | Z |
A | Zn | Cu | ϡ���� |
B | Cu | Zn | ϡ���� |
C | Cu | Ag | ����ͭ��Һ |
D | Ag | Zn | ��������Һ |
A.AB. BC. CD. D