��Ŀ����
����Ŀ����ɫ��ѧ��Ԥ����Ⱦ�ĸ����ֶΣ�����Ŀ�����о���Ѱ���ܳ�����õ�����ԭ���ϣ�����ȵؽ�Լ��Դ���ڻ����������������ж�ʵ�־���������Ⱦ��
(1)���и�����ϡ���ɫ��ѧ��Ҫ�����________������ţ���
A.���������� B.������Ⱦ�� C.�����ж��� D.�ž���ȾԴ
(2)ij�������ŷŵ���ˮ�к���![]() ��
��![]() ��
��![]() ��
��![]() �������ӡ�ijͬѧ����˴Ӹ���ˮ�л��ս���ͭ�ķ�����
�������ӡ�ijͬѧ����˴Ӹ���ˮ�л��ս���ͭ�ķ�����
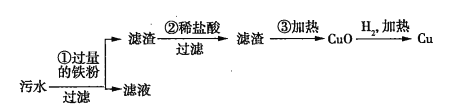
�ڻ��ս���ͭ�ķ����У�����_______�ᵼ�»�����Ⱦ��Ϊ��ֹ��Ⱦ��Ӧ��ȡ�Ĵ�ʩ��_______________________________________��
���𰸡�D �� ������������װ��
��������
(1)���������������ɫ��ѧ��Ԥ����Ⱦ�ĸ����ֶΡ���֪������ɫ��ѧ��Ҫ��ӡ�Դͷ���϶ž���ȾԴ����ѡ D ��
(2)���ڹ��ķе�͡��ӷ����ж�����˲����ۻ���ɻ�����Ⱦ��Ӧ��ȡ�Ĵ�ʩ��������������װ�á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����1����������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ����1L�ܱ������У���ʼͶ��4mol N2��6mol H2��һ������������NH3��ƽ��ʱ���ı��¶Ȳ�õ����������ʾ ����֪��T1<T2��
�¶� | ƽ��ʱNH3�����ʵ���/mol |
T1 | 3.6 |
T2 | 2 |
����K1_______K2 ����������������������=������ԭ��__________��
����T2�£�����10s�ﵽ��ѧƽ��״̬����ƽ��ʱH2��ת����Ϊ______________������ͬʱ���Ӹ����ʵ���Ϊ1mol����÷�Ӧ������v��_____v��������=����ƽ�ⳣ����______������������������С���������䣩��
��2��һ���¶��£���3mol A�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��
3A��g��+B��g��![]() xC��g��������д���пհף�
xC��g��������д���пհף�
�ٷ�Ӧ1minʱ���ʣ��1.8mol A��C��Ũ��Ϊ0.4 mol/L����1min��B�ķ�Ӧ����Ϊ______��xΪ_______��
�������������ʼѹǿΪP0��10min���ƽ�⣬�����ڻ��������ѹǿΪP����P0��P����ʾ��ƽ��ʱ��Ӧ��A��ת����a��A��Ϊ__________��
���ܹ�˵���÷�Ӧ�ﵽƽ��ı�־��___________��
A �����ڻ��������ܶȱ��ֲ���
B v��A��=3v��B��
C A��B��Ũ��֮��Ϊ3:1
D ��λʱ��������3 n molA��ͬʱ����n mol B
E ��ϵ���¶Ȳ��ٱ仯
����Ŀ����H2O2��KI��ϴ�ྫ�����������������ʵ�飨��ʱ���ڲ���������ĭ����ijͬѧ�����������϶Ը�ʵ�����̽����
(1)����1��KI�ڸ÷�Ӧ�е����ã�H2O2 + I![]() = H2O + IO
= H2O + IO![]() ��H2O2 + IO
��H2O2 + IO![]() = H2O + O2��+ I
= H2O + O2��+ I![]() ���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
(2)����2��H2O2�ֽⷴӦ�����������仯��ͼ��ʾ�����Т���KI���룬����KI���롣�����ж���ȷ����______������ĸ����
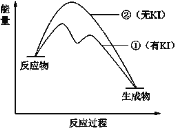
a������KI��ı��˷�Ӧ��·��
b������KI��ı����ܷ�Ӧ�������仯
c��H2O2 + I![]() = H2O + IO
= H2O + IO![]() �Ƿ��ȷ�Ӧ
�Ƿ��ȷ�Ӧ
(3)ʵ���з��֣�H2O2��KI��Һ��Ϻ����������ݣ���Һ��ɫ��ơ��ټ���CCl4�������ã��������Լ��١�
����3��I2Ҳ�ɴ�H2O2�ķֽⷴӦ��
�� ��CCl4�������úɹ۲쵽___________________________________��˵����I2���ɡ�
�� �������Լ��ٵ�ԭ������ǣ�
��. H2O2Ũ�Ƚ��ͣ�
��._________________________________________��
���¶���ʵ��˵����������Ҫԭ����H2O2��Һ�м���KI��Һ������Һ��ƺֳ����ȷ���A��B���Թ��С�A�Թܼ���CCl4��B�Թܲ���CCl4���ֱ������á��۲쵽��������________________________��
(4)����4��I![]() + I
+ I![]()
![]() I
I![]() K= 640��
K= 640��
Ϊ��̽����ϵ�к������Ĵ�����ʽ������ʵ�飺��20 mLһ��Ũ�ȵ�H2O2��Һ�м���10 mL 0.10 mol��L-1 KI��Һ����ƽ��������Ũ�����£�
�� | I | I | I |
Ũ��/ (mol��L-1) | 2.5��10-3 | a | 4.0��10-3 |
�� a =____________________��
�� ��ƽ����ϵ�г��˺���I![]() ��I
��I![]() ��I
��I![]() �⣬һ��������������������������_____________________��
�⣬һ��������������������������_____________________��