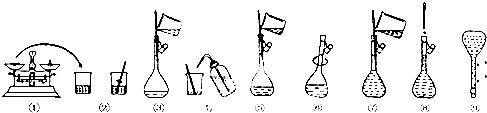��Ŀ����
20����ˮ�Ǿ����Դ���⣬��ˮ���������ۺ����þ�����Ҫ���壮
���������գ�
��1���ȼҵ��Ҫ��ʳ��Ϊԭ�ϣ�Ϊ�˳�ȥ�����е�Ca2+��Mg2+��SO42-����ɳ���ɽ���������ˮ��Ȼ��������в�������ȷ�IJ���˳����AD��
�ٹ��ˣ��ڼӹ�����NaOH��Һ���ۼ�����������ܼӹ�����Na2CO3��Һ���ݼӹ�����BaCl2��Һ
A���ڢݢܢ٢�B���٢ܢڢݢ�C���ܢڢݢ٢�D���ݢڢܢ٢�
��2����ʵ�����п�������ȡ�ķ�����ȡ�壬��ѡ�õ��Լ���CCl4��������Ҫ�����������Ƿ�Һ©����
��3����������������ữ�����Cl2�����ʵ�ԭ�����ữ������Cl2��Br2��ˮ��Ӧ��
��4������II��Ӧ�����ӷ���ʽBr2+SO2+2H2O=4H++SO42-+2Br-��
��5����ˮ������������У��¶�Ӧ������80��90�棬�¶ȹ�����Ͷ������������������ԭ���¶ȹ��ߣ�����ˮ������֮�ų���������ˮ�����ӣ��¶ȹ��ͣ��岻����ȫ���������ʵͣ�
��6��Mg��OH��2�����л���Ca��OH��2����ѡ��MgCl2��Һ����ϴ�ӳ�ȥ����ֱ�Ӽ���Mg��OH��2�õ�MgO���ٵ������MgO�ƽ���þ�������ɼ�ʵ�鲽�裬�㲻ͬ�⣨ѡ�ͬ�⡱������ͬ�⡱����˵��������MgO�۵�ܸߣ�����ʱ���ܸߣ����������ɱ�����ע������þɾȥ�۵�2852�棬�е�3600���Ȼ�þɾȥ�۵�714�棬�е�1412�棩
���� ���̷�����ˮɹ�κ�õ����Σ���ˮ�������ᴿ��ȥ��������Ca2+��Mg2+��SO42-����ɳ���õ����Σ�����Ʊ�������ĸҺ����Ҫ�������Ӻ�þ���ӣ�ͨ����������������Ϊ�嵥�ʣ�������Ͷ�����������ͨ��ˮ�еõ�±ˮ��Һ���廯����Һ�������壬�������������廯��Ϊ�嵥�ʣ����Ȼ�þ����Һ�м��뱴�Ǹ��·ֽ����ɵ�����������ˮ���ɵ���������ʯ���飬����þ���ӣ����˺��ڳ����м��������ܽ⣬ͨ��Ũ����������ȴ�ᾧ����ϴ�ӵõ��Ȼ�þ���壬ʧˮ�õ��Ȼ�þ���壬���õ�þ��
��1������ʱΪ�˰����ʳ��������Լ����������ȥ�����е�Ca2+��Mg2+��SO42-�ֱ�ѡ��Na2CO3��Һ��NaOH��Һ��BaCl2��Һ��ǰ�����Լ������������������ȥ��BaCl2��Һֻ�ܷ��ڼ���Na2CO3��Һ����ǰ�棬�����ȥ��ע�������ǰ���˳�ȥ�������ʲ���˳��Ϊ�ڢݢܢ٢ۻ�ݢڢܢ٢ۣ�
��2����ȡ�Լ�������ˮ�����ұ���ȡ���������Լ��е��ܽ��ҪԶ������ˮ�е��ܽ�ȣ�ʵ���ڷ�Һ©���н��У�
��3��Cl2��Br2��ˮ��Ӧ�ǿ��淴Ӧ���ữ������Cl2��Br2��ˮ��Ӧ��
��5���¶ȹ��ߣ�����ˮ������ˮ�ų�������������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵͣ�
��6��ѡ��MgCl2��Һ��ʹCa��OH��2ת��ΪMg��OH��2��ͬʱϴȥ���ʣ�MgO�۵�ܸߣ�����ʱ���ܸߣ����������ɱ����ʲ��õ������MgO�ƽ���þ��
��� �⣺��1��Ca2+��̼����ת��Ϊ������Mg2+��NaOHת��Ϊ������SO42-���Ȼ�����Һת��Ϊ������Ȼ�������ù��˷�����ȥ���г����������ȳ�ȥMg2+��SO42-��Ȼ���ٳ�ȥCa2+����Ϊ̼������̼��������ױ������ȥ�������� �������ƻ��Ȼ���֮���ټ�̼������Һ�����˺��ټ������ȥ�����ġ�NaOH��̼���ƣ����Գ���˳���Ǣڢݢܢ٢ۻ�ݢڢܢ٢ۣ�
��ѡAD��
��2������CCl4���е��ܽ�ȴ�����ˮ��Һ�е��ܽ�ȣ����������ʺ��嶼����Ӧ����ˮ�����ܣ�����ѡȡ��ȡ��ΪCCl4����ȡʹ������Ϊ��Һ©����
�ʴ�Ϊ��CCl4����Һ©����
��3��Cl2+H2O?HCl+HClO��Br2+H2O?HBr+HBrO�������ữʱ����Һ��������Ũ����������Cl2��Br2��ˮ��Ӧ�������������ữ�����Cl2�����ʣ�
�ʴ�Ϊ���ữ������Cl2��Br2��ˮ��Ӧ��
��4�������ǿ�����ԣ�����������л�ԭ�ԣ�������ˮ��Һ���������ԭ��Ӧ��������������ᣬ���ӷ�Ӧ����ʽΪBr2+SO2+2H2O=4H++SO42-+2Br-��
�ʴ�Ϊ��Br2+SO2+2H2O=4H++SO42-+2Br-��
��5������ˮ�У���ķе���58.5��C��ˮ����100��C���¶ȹ��ߣ�����ˮ������֮�ų���������ˮ�����ӣ��¶ȹ��ͣ��岻����ȫ���������ʵͣ�
�ʴ�Ϊ���¶ȹ��ߣ�����ˮ������֮�ų���������ˮ�����ӣ��¶ȹ��ͣ��岻����ȫ���������ʵͣ�
��6��Ca��OH��2�������Mg��OH��2���������Mg��OH��2�����л���Ca��OH��2����MgCl2��Һ����ϴ�ӣ�ʹCa��OH��2ת��Ϊ������Mg��OH��2�Ӷ���ȥCa��OH��2��MgO�۵�ܸߣ������������þ��Ҫ���Ľϸߵ��������Ӷ����ӳɱ�����ҵ���õ�������Ȼ�þұ��Mg��
�ʴ�Ϊ��MgCl2����ͬ�⣻MgO�۵�ܸߣ�����ʱ���ܸߣ����������ɱ���
���� ���⿼���˺�ˮ��Դ�������ã��漰������ұ�������ʵķ�����ᴿ��������ԭ��Ӧ��֪ʶ�㣬�ܴ������Ϸ�����������ͼ��֪��ÿһ�������ķ�Ӧ�������������Ϥ�������ʵķ��뷽����ע�⣨1���г��Ӽ��μ�˳��Ϊѧϰ�״��㣬��Ŀ�Ѷ��еȣ�

 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�| A�� | v��NH3��=0.6 mol•L-1•min-1 | B�� | v��N2��=0.005 mol•L-1•s-1 | ||
| C�� | v��H2��=0.9 mol•L-1•min-1 | D�� | v��NH3��=0.02 mol•L-1•s-1 |
| A�� | O2��O3 | B�� | ${\;}_{1}^{1}$H��${\;}_{1}^{2}$H | ||
| C�� | CH3CH3��CH3CH2 CH3 | D�� | CH3CH2CH2CH3��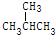 |
| A�� | �������������������NaOH��Һ��Al2O3+2OH-+3H2O�T2Al��OH��3�� | |
| B�� | ̼�����Ƶ�ˮ�⣺HCO3-+H2O?H3O++CO32- | |
| C�� | 500�桢30MPa�£���0.5mol N2��1.5mol H2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6KJ/mol | |
| D�� | ����ı�ȼ����Ϊ-890.3kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��1����H=-890.3kJ•mol-1 |
| A�� | �Ȼ�����Һ�ڵ��������µ���������Ӻ������ӵĹ��̽е��� | |
| B�� | ������ˮ���ܵ���������ӵĻ����ﶼ���� | |
| C�� | ������̼����ˮ���ܵ��磬�ʶ�����̼���ڵ���� | |
| D�� | ���ᱵ������ˮ�����ܽⲿ����ȫ���룬�����ᱵ��ǿ����� |