��Ŀ����
����Ŀ��ij�о���ѧϰС������������о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ���������ʵ��̽��������ͬ�ش��������⣺
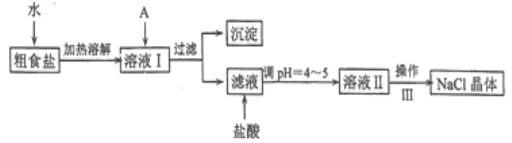
̽��һ�����ͼ��ʾװ�ý���������ˮ��Ӧ����ʵ��(�г�������)��
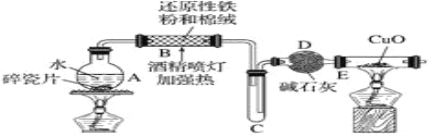
(1)Ӳ�ʲ�����B�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
(2)��ӦǰA��Ͷ�����Ƭ��Ŀ����_______��
(3)װ��E�е�������____________��
̽�����������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ�����B�к�ɫ����ijɷ֡�
(4)��Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������___��������Һ�ֳ����ݡ�
(5)һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ____ (����ţ���ͬ)������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ______��
��һ����Fe3O4����һ����Fe ��ֻ��Fe3O4������ֻ��Fe
(6)��һ���ý�ͷ�ιܼ���_______(���Լ�������)������֤����Һ�д���Fe2����
̽��������������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������
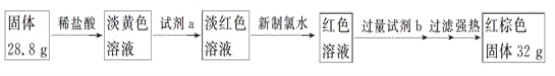
(7)�Լ�b�Ļ�ѧʽ��____________��
(8)���㷴Ӧ��Bװ������Ԫ�ص���������Ϊ_______��
���𰸡�3Fe��4H2O(g)![]() Fe3O4��4H2 ��ֹ���� ��ɫ�����죬�Ҷ˹ܱ���ˮ�� ϡ���� �� �� ����KMnO4��Һ����Һ��ɫ(�����軯����Һ��������ɫ�������𰸺�������) ��Һ����NaOH��KOH) 77.8%
Fe3O4��4H2 ��ֹ���� ��ɫ�����죬�Ҷ˹ܱ���ˮ�� ϡ���� �� �� ����KMnO4��Һ����Һ��ɫ(�����軯����Һ��������ɫ�������𰸺�������) ��Һ����NaOH��KOH) 77.8%
��������
(1)Ӳ�ʹ�������ˮ�����ڸ����·�Ӧ����������������������
(2)Һ�����ʱ�������������������Ƭ�������Ƿ�ֹ���С�
(3)װ��E�з�����ӦΪH2��CuO![]() Cu��H2O����Ӧ�����Ǻ�ɫ�����죬�Ҷ˹ܱ���ˮ�顣
Cu��H2O����Ӧ�����Ǻ�ɫ�����죬�Ҷ˹ܱ���ˮ�顣
(4)����֤��Ӧ���ɫ����ijɷ�ʱ������Fe3��������Լ�ΪKSCN��Һ����ȷ������Fe3��ʱ������Fe2������������KMnO4��Һ����������KMnO4��Һ�����ᷢ����Ӧ���������ܽⷴӦ��ĺ�ɫ����ʱ�����������ᣬҲ����������(��ΪHNO3������Fe2��)������ϡ���ᡣ
(5)һ�ݵμӼ���KSCN��Һ������Һ���ɫ��˵����Һ�к��������ӣ�����ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ��һ����Fe3O4������Һδ���ɫ������Һ�в����������ӣ����ƶ�Ӳ�ʲ�����B�й���������һ������Fe��������ȷ��
(6)�����������ӣ���Ҫ�ý�ͷ�ιܼ������Ը��������Һ�������Ը��������Һ��ɫ����֤����Һ�к����������ӡ�
(7)��������ɫ������֪��������ΪFe2O3�����Լ�bΪNaOH��Һ��
(8)������������Ϊ��m(Fe2O3)��32 g���������������ʵ���Ϊn(Fe2O3)��![]() ��0.2 mol��������Ԫ�ص����ʵ���Ϊn(Fe)��0.4 mol�������㷴Ӧ��Bװ������Ԫ�ص�����������
��0.2 mol��������Ԫ�ص����ʵ���Ϊn(Fe)��0.4 mol�������㷴Ӧ��Bװ������Ԫ�ص�����������
(1)Ӳ�ʹ�������ˮ�����ڸ����·�Ӧ������������������������Ӧ�Ļ�ѧ����ʽΪ3Fe��4H2O(g)![]() Fe3O4��4H2���ʴ�Ϊ��3Fe��4H2O(g)
Fe3O4��4H2���ʴ�Ϊ��3Fe��4H2O(g)![]() Fe3O4��4H2��
Fe3O4��4H2��
(2)Һ�����ʱ�������������������Ƭ�������Ƿ�ֹ���У��ʴ�Ϊ����ֹ���С�
(3)װ��E�з�����ӦΪH2��CuO![]() Cu��H2O����Ӧ�����Ǻ�ɫ�����죬�Ҷ˹ܱ���ˮ�飬�ʴ�Ϊ����ɫ�����죬�Ҷ˹ܱ���ˮ�顣
Cu��H2O����Ӧ�����Ǻ�ɫ�����죬�Ҷ˹ܱ���ˮ�飬�ʴ�Ϊ����ɫ�����죬�Ҷ˹ܱ���ˮ�顣
(4)����֤��Ӧ���ɫ����ijɷ�ʱ������Fe3��������Լ�ΪKSCN��Һ����ȷ������Fe3��ʱ������Fe2������������KMnO4��Һ����������KMnO4��Һ�����ᷢ����Ӧ���������ܽⷴӦ��ĺ�ɫ����ʱ�����������ᣬҲ����������(��ΪHNO3������Fe2��)������ϡ���ᣬ�ʴ�Ϊ��ϡ���ᡣ
(5)һ�ݵμӼ���KSCN��Һ������Һ���ɫ��˵����Һ�к��������ӣ�����ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ��һ����Fe3O4������Һδ���ɫ������Һ�в����������ӣ����ƶ�Ӳ�ʲ�����B�й���������һ������Fe��������ȷ���ʴ�Ϊ����������
(6)�����������ӣ���Ҫ�ý�ͷ�ιܼ������Ը��������Һ�������Ը��������Һ��ɫ����֤����Һ�к����������ӣ��ʴ�Ϊ������KMnO4��Һ����Һ��ɫ(�����軯����Һ��������ɫ�������𰸺�������)��
(7)��������ɫ������֪��������ΪFe2O3�����Լ�bΪNaOH��Һ���ʴ�Ϊ����Һ����NaOH��KOH)��
(8)������������Ϊ��m(Fe2O3)��32 g���������������ʵ���Ϊn(Fe2O3)��![]() ��0.2 mol��������Ԫ�ص����ʵ���Ϊn(Fe)��0.4 mol����Ӧ��Bװ������Ԫ�ص���������Ϊ
��0.2 mol��������Ԫ�ص����ʵ���Ϊn(Fe)��0.4 mol����Ӧ��Bװ������Ԫ�ص���������Ϊ![]() ��100%��77.8%�����ʴ�Ϊ��77.8%��
��100%��77.8%�����ʴ�Ϊ��77.8%��

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�