��Ŀ����
����Ŀ����Ӧ4HCl(g)��O2(g)![]() 2Cl2(g)��2H2O(g)�У�4 mol HCl���������ų�115.6 kJ����������֪��
2Cl2(g)��2H2O(g)�У�4 mol HCl���������ų�115.6 kJ����������֪��![]() ���ж�����˵����ȷ����( )
���ж�����˵����ȷ����( )
A. �÷�Ӧ�Ħ�H����115.6 kJ��mol��1
B. �Ͽ�1 mol H��O ����Ͽ�1 mol H��Cl �������������ԼΪ32 kJ
C. H2O��H��O ����HCl��H��Cl����
D. �����ṩ�����ж���Ԫ�صķǽ����Ա���Ԫ��ǿ
���𰸡�B
��������
A�����ݷ�ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ���Ȼ�ѧ����ʽΪ��4HCl(g)+O2(g)2Cl2(g)+2H2O(g)��H=-115.6 kJ/mol����A����B��E(H-O)��E(HCl)�ֱ��ʾH-O���ܡ�H-Cl���ܣ���ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ����H=��Ӧ���ܼ���-��������ܼ��ܣ��ʣ�4��E(H-Cl)+498kJ/mol-[2��243kJ/mol+4��E(H-O)]=-115.6kJ/mol�������ã�4E(H-Cl)-4E(H-O)=-127.6kJ/mol����E(H-O)-E(HCl)=31.9kJ/mol���ʶϿ�1mol H-O����Ͽ�1mol H-Cl�������������ԼΪ31.9kJ/mol��1mol=31.9kJ����B��ȷ��C��E(H-O)��E(HCl)�ֱ��ʾH-O���ܡ�H-Cl���ܣ���ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ����H=��Ӧ���ܼ���-��������ܼ��ܣ��ʣ�4��E(H-Cl)+498kJ/mol-[2��243kJ/mol+4��E(H-O)]=-115.6kJ/mol�������ã�4E(H-Cl)-4E(H-O)=-127.6kJ/mol����E(H-O)-E(HCl)=31.9kJ/mol���ʶϿ�1mol H-O����Ͽ�1mol H-Cl�������������ԼΪ31.9kJ/mol��1mol=31.9kJ��32kJ��H2O��H-O����HCl��H-Cl��ǿ����C����D������C�ķ�����֪��H2O��H-O����HCl��H-Cl��ǿ��˵��H-O����H-Cl���ȶ������ǽ�����O��Cl����D����ѡB��

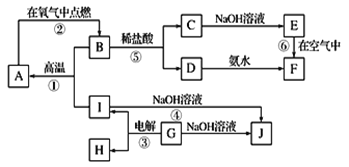
����Ŀ����������������п�̸ɵ�صĻ������ϣ���ҵ�������̿����̿�Ϊԭ�����Ʊ���ij���̿���Ҫ�ɷ�ΪMnO2��������Si��16.27%����Fe��5.86%����Al��3.42%����Zn��2.68%����Cu��0.86%����Ԫ�صĻ�����䴦������ͼ���£�

������ | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
Ksp����ֵ | 10-35 | 10-6 | 10-38 |
��1���������������������½�MnO2��ԭΪMnSO4�����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ______________________��
��2������ˮ���������������������������ܼӿ췴Ӧ���ʣ�����___________������A�ijɷ���Fe��OH��3��Al��OH��3�����백ˮ�����pH ���ٴﵽ____________��ǡ����ʹFe3+��A13+������ȫ��(������Ũ�Ƚ���1.0��10-5mol��L-1ʱ����Ϊ������ȫ)
��3������B�ijɷ���___________________��
��4��MnO2Ҳ����MnSO4-H2SO4-H2OΪ��ϵ�ĵ��Һ�е���ã��������缫��ӦʽΪ_____________________________________________________��
��5����ҵ�ϲ��ü��������ԭ�ζ����ⶨMnO2���ȣ�������������£�ȷ����0.9200g����Ʒ������������KI ��Һ��ַ�Ӧ�����Ƴ�100mL��Һ��ȡ����20.00mL��ǡ����25.00mL 0.0800mol��L-1Na2S2O3��Һ��Ӧ��I2+2S2O32-=2I-+S4O62-��������ɵø���Ʒ����_____%��(С�������1λ����)��
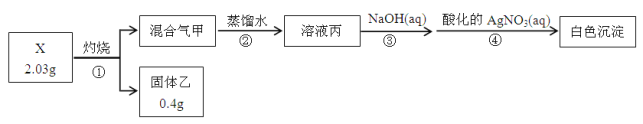
����Ŀ��ij�غ����к���Ca2����Mg2����Fe3����SO42�����������ӣ�����øõغ�����ȡ�ռ�����Ƶþ���ʳ��ˮ�Ĺ������£�

��1�����̢��н����μ�ˮ�ܽ���Ҫ�ʵ����ȣ���Ŀ����__________��
��2�����̢��Ŀ���dz�ȥSO42���������X��Һ��__________��
��3���±��ǹ��̢������ɵIJ��ֳ���������20��ʱ���ܽ��[g/100gH2O]
CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 | Fe(OH)3 |
2.6��10��2 | 2.5��10��4 | 7.8��10��4 | 2.4��10��4 | 1.7��10��3 | 4.8��10��9 |
�ٹ��̢������ɵ���Ҫ������CaCO3��Fe(OH)3���__________��
�ڹ��̢��е���pHʱ��������Ҫ��Ӧ�����ӷ���ʽΪ__________��
��4����������ʳ��ˮ�л���������I����IO3����NH4����Ca2����Mg2������ȥ��Щ���Ӽ����е����������£�
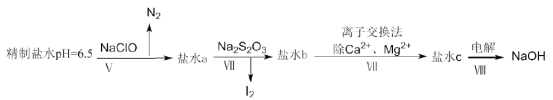
�ٹ���V����N2�����ӷ���ʽΪ__________��
�ڹ��̢�����ͨ������Na2S2O3��������IO3����ԭ��I2������ˮb�к���SO42�����ù����з���������ԭ��Ӧ�������������ͻ�ԭ�������ʵ���֮��Ϊ__________��
���ڹ��̢������õ�Na2S2O3�׳ƺ�������һ����Ҫ�Ļ���ԭ�ϡ���Ʒ������Ҫ�ɷ���Na2S2O3��5H2O��Ϊ�˲ⶨ�京Na2S2O3��5H2O�Ĵ��ȣ���ȡ8.00g��Ʒ�����250mL��Һ��ȡ25.00mL����ƿ�У��μӵ�����Һ��ָʾ��������Ũ��Ϊ0.0500mol��L��1�ĵ�ˮ�ζ���������Ӧ2S2O32����I2��S4O62����2I�������±���¼�ζ������
�ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
��һ�� | 0��30 | 29��12 |
�ڶ��� | 0��36 | 30��56 |
������ | 1��10 | 29��88 |
������Ʒ�Ĵ���Ϊ__________��