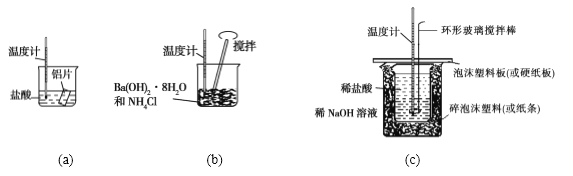
��Ŀ����
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�顣
[����ʽ��ȷ��]
(1)���л���A�����������г��ȼ�գ�ʵ��������5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������___��
(2)�����Dzⶨ�л����������Է�������Ϊ46��������ʵķ���ʽ��____��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ____��
[�ṹʽ��ȷ��]
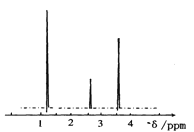
(4)���ⶨ���л���A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ___��
[����ʵ��]
(5)A��Cu�����¿ɱ���������B���仯ѧ����ʽΪ_______��
���𰸡�2��6��1 C2H6O CH3CH2OH��CH3OCH3 CH3CH2OH 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O
��������
(1)����ˮ��������������̼����������ȷ���л�����C��Hԭ�ӵ����ʵ�����������ĵ�O2�����������л�����OԪ�ص�������������ȷ���л����и�ԭ�Ӹ�����ֵ��
(2)�����л���ԭ�Ӹ�����ֵ��ȷ�����ʽ�������Է���������ȷ���л������ʽ��
(3)�����л������ʽ��ϼۼ����ۿ�ȷ���л���Ŀ��ܽṹ��
(4)�����л�������к��е�Hԭ�ӹ���ͼȷ���л���Ľṹ��
(5)AΪ�Ҵ���һ�������¿�����������ȩ���ݴ���д��Ӧ����ʽ��
(1)�������֪n(H2O)= 5.4g��18g/mol=0.3mol��n(CO2)=8.8g��44g/mol=0.2mol��n(O2)=0.67L��22.4L/mol=0.3mol��������ԭ���غ��֪�л����к���n(O)=0.3mol+0.2mol��2-0.3mol��2=0.1mol�����л�����N(C)��N(H)��N(O)=0.2mol��0.6mol��0.1mol=2��6��1��
(2)�������и�Ԫ�ص�ԭ�Ӹ�����ΪN(C)��N(H)��N(O)=2��6��1�������ʽΪC2H6O������Է�������Ϊ46�����л���ķ���ʽΪC2H6O��
(3)�л���ķ���ʽΪC2H6O�������п��ܴ���C-C��C-H��C-O��O-H�Ȼ�ѧ�������ܵĽṹ��ʽ��CH3CH2OH��CH3OCH3��
(4)�л���A�����������ֻ�ѧ��������ԭ�ӣ�ӦΪ�Ҵ�����CH3CH2OH��������ֻ��һ�ֲ�ͬ��ѧ��������ԭ�ӣ�
(5)�Ҵ���Cu�������±���������CH3CHO���÷�Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��

����Ŀ��ʵ�顢��ȡ������ǻ�ѧѧϰ����Ҫ�����������й��̵������ȷ����![]()
ʵ�� | ���ʵ�� | |
A |
| �� |
B | ��FeͶ�������Ũ�����в��������Ա仯 | ��CuͶ�������Ũ����Ҳ���ᷴӦ |
C |
|
|
D | ��ȩ����ʹ�� | ��ȩҲ��ʹ����ˮ��ɫ |
A.AB.BC.CD.D
����Ŀ���ȱ���Ⱦ�ϡ�ҽҩ���л��ϳɵ��м��壬����Ҫ���л�������Ʒ��ʵ������ȡ�ȱ���װ����ͼ��ʾ(���Ⱥ̶�������װ������ȥ)��
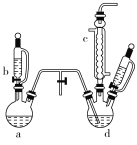
�ش��������⣺
(1)�����a��b������ϳ���ȡ������װ�ã���Ӧ������ȣ���a�����еĹ��巴Ӧ�������________(�����)��
A.MnO2����������������B.KMnO4 C.K2Cr2O7
(2)����c��������________��
(3)��ȡ�ȱ��Ļ�ѧ����ʽΪ________________��
(4)����d�еķ�Ӧ��ɺ�ҵ��Ҫ����ˮϴ����ϴ��ʳ�θ����������ϴ֮ǰҪ����ˮϴ����Ŀ����______________________��
(5)���ɵ������д���HCl��H2O���������壬����Ҫ����һ��װ�ó�ȥˮ�����������¿��л����������������Լ���
__________
(6)��ҵ�����б�����ʧ��������ʾ��
��Ŀ | ���ȱ� | �Ȼ�β�� | ���� | ��Ʒ | ��ȷ������ | �ϼ� |
����ʧ��/(kg��t-1) | 11.7 | 5.4 | 20.8 | 2.0 | 49.3 | 89.2 |
��10 t�����Ƶó�Ʒ�ȱ�________t��(�г�����ʽ���ɡ��ȱ��ͱ�����Է��������ֱ���112.5��78)
����Ŀ��ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����͡������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��С�
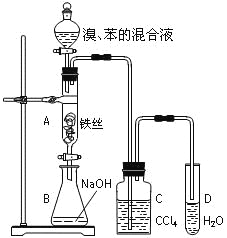
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ��________________________________________��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_________________________��
��3��C��ʢ��CCl4��������_____________________________��
��4����Ҫ֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ��ͨ�������ַ������밴Ҫ����д�±���
���Թ�D�м�����Լ� | ��֤������Һ�巢��ȡ����Ӧ������ | |
����һ | _____________ | _________________ |
������ | _____________ | _________________ |