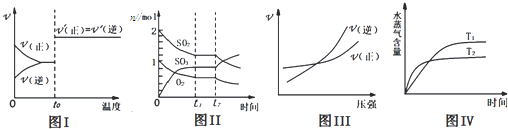
题目内容
【题目】已知![]() N2(g)+
N2(g)+![]() H2(g)=N(g)+3H(g) ΔH1=+akJ·mol-1;
H2(g)=N(g)+3H(g) ΔH1=+akJ·mol-1;
N(g)+3H(g)=NH3(g) ΔH2=-bkJ·mol-1;
NH3(g)=NH3(l) ΔH3=-ckJ·mol-1
试写出N2(g)和H2(g)反应生成液氨的热化学方程式:__。
【答案】N2(g)+3H2(g)=2NH3(l) ΔH=-2(b+c-a)kJ·mol-1
【解析】
①![]() N2(g)+
N2(g)+![]() H2(g)=N(g)+3H(g) ΔH1=+akJ·mol-1;
H2(g)=N(g)+3H(g) ΔH1=+akJ·mol-1;
②N(g)+3H(g)=NH3(g) ΔH2=-bkJ·mol-1;
③NH3(g)=NH3(l) ΔH3=-ckJ·mol-1;
根据盖斯定律,①×2+②×2+③×2得N2(g)+3H2(g)=2NH3(l) ΔH=2ΔH1+2ΔH2+2ΔH3=-2(b+c-a)kJ·mol-1,所以N2(g)和H2(g)反应生成液氨的热化学方程式是N2(g)+3H2(g)=2NH3(l) ΔH=-2(b+c-a)kJ·mol-1。

练习册系列答案
 应用题天天练四川大学出版社系列答案
应用题天天练四川大学出版社系列答案
相关题目
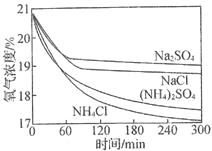
【题目】利用如下实验探究铁钉在不同溶液中的吸氧腐蚀。下列说法不正确的是
实验装置 | 实验编号 | 浸泡液 | pH | 氧气浓度随时间的变化 |
|
|
| 5 |
|
|
| 5 | ||
|
| 7 | ||
|
| 7 |
A.上述正极反应均为![]()
B.在不同溶液中,![]() 是影响吸氧腐蚀速率的主要因素
是影响吸氧腐蚀速率的主要因素
C.向实验![]() 中加入少量
中加入少量![]() 固体,吸氧腐蚀速率加快
固体,吸氧腐蚀速率加快
D.在![]() 内,铁钉的平均吸氧腐蚀速率酸性溶液大于中性溶液
内,铁钉的平均吸氧腐蚀速率酸性溶液大于中性溶液